
Optimizing outcomes of anterior cruciate ligament (ACL) reconstruction in female athletes: from graft choice to return to sport criteria
Introduction
Anterior cruciate ligament (ACL) injury is common in female athletes with a reported incidence up to nine times more often compared to males in similar cutting and pivoting sports (1,2). While there are high success rates with ACL reconstruction (ACLR), females, younger, and more active patients are at risk for re-injury of the index or contralateral knee (3-6). The postoperative rehabilitation after ACLR and return to sport (RTS) can take 8–12 months (7,8) and studies demonstrate that females have a lower likelihood to RTS at the same level compared to males (9). Given that females have: (I) an increased risk of ACL injury, (II) a higher risk of re-tear after ACLR and (III) a lower rate of RTS, it is paramount to surgically and postoperatively try to mitigate these risks.
Graft choice: type, diameter, and surgical technique
First and foremost, surgical technique should be scrutinized to ensure that graft choice, graft diameter, graft fixation and tunnel placement is optimized for each individual female patient to minimize the risk of re-tear, injury to the contralateral knee, and to maximize the chance of RTS at the preinjury level. Graft choice must be carefully considered to ensure an appropriately sized graft in female athletes who often may have smaller anatomy compared to a male.
There have been many studies that have compared the re-tear rates and joint laxity of autologous bone-patellar tendon-bone (BTB) and hamstring (HS) grafts in ACLR (1,10). Results of these studies vary regarding both factors. A study by Salem et al. indicates that BTB grafts show fewer ruptures in 15–20 year old females, but this result is not seen in females ages 21–25 years old (1). Another study demonstrates that there are small and mostly insignificant differences in long-term follow-up between these two graft types in regard to re-tear and joint laxity (11). One difference commonly seen between these two grafts is the significant increase in kneeling pain especially in females with a BTB graft when compared to HS grafts (1,11). In a systematic review of twelve studies, no difference between BTB and HS grafts was found with regard to graft failure (10). The risk of osteoarthritis (OA) was significantly higher in BTB patients in the majority of the studies reviewed, however there is evidence that both graft types lead to a higher chance of developing OA in the index knee than in a healthy or contralateral knee (10,11). Female athletes, prone to anterior and kneeling pain, should be advised regarding the risks of ACLR with BTB and HS grafts should be considered for use in this population.
ACLR graft ruptures are often seen with grafts of inadequate diameter. Insuring that optimal graft length and diameter are obtained is essential to minimizing the risks of failure and need for revision (12-14). Recent literature has shown that a minimum of an 8mm graft is necessary to decrease the likelihood of a revision due to graft rupture, especially in younger and female patients (13,15-20). Other studies have shown that grafts less than 9 mm in diameter should be avoided to decrease risk of re-tear (21). To further show the importance of adequate graft diameter, one study determined that the likelihood of a patient needing revision ACLR in their study cohort was 0.82 times lower for every 0.5-mm increase in the graft diameter from 7.0 to 9.0 mm (22) (Table 1).
Table 1
| Author (ref) | # of patients, M:F | Graft type | Mean graft diameter | Outcomes |
|---|---|---|---|---|
| Bjornsson 2016, ( |
193, 131:62 | 61 BTB, 86 HS | – | No difference in re-tear rate |
| Nguyen 2016, ( |
503, 235:268 | Quadrupled HS autograft | 7.9 mm | Re-tear rate 6% (28M;17F); graft size <8 mm and age <25 had increased risk of re-tear |
| Spragg 2016, ( |
491, 259:232 | HS autograft | 8.1 mm controls; 7.9 mm requiring revision | 132 revisions (M65:F59); likelihood of re-tear was 0.82× lower for every 0.5 mm increase in graft diameter from 7–9 m |
| Schurz 2016, ( |
79, 53:26 | Quadrupled HS | 8.3 mm (range, 8.27–11 mm); 8.0 mm in 10 failed grafts (range, 6.5–9.5 mm) | 12% re-rupture at mean of 17.6 months due to sports injury; no difference in diameter between ruptured grafts and intact grafts |
| Yasen 2017, ( |
108, 81:27 | Quadrupled HS | 8.5 mm | 6.5% re-tear due to trauma |
| Kaeding 2017, ( |
2,497, 1,368:1,129 | BTB 1,132, HS 891, allograft 460 | – | Re-tear: 112 ipsilateral, 90 contralateral; allograft 13.13× > BTB autograft: no difference between BTB and HS; no correlation between sex and re-tear rate |
| Salem 2019, ( |
256 F | 175 BTB, 81 HS | >8 mm (18 HS augmented with allograft because <8 mm) | 12 BTB re-tear; 11 HS re-tear of which 4 had allograft augmentation; 62.7% returned to preinjury level of sport |
| Desai 2019, ( |
136, 80:56 | 82 quadrupled HS all-inside (49M:33F); 54 HS complete tibial tunnel (31M:23F) | All-inside 9.0 mm; tibial tunnel 8.3 mm | All inside: 8 graft failure, RTS 12.5 months, Tegner score 6.4; tibial tunnel: 10 graft failure, RTS 9.9 months, Tegner score 6.8; M |
BTB, bone-patellar tendon-bone; HS, hamstring; RTS, return to sport; ACLR, anterior cruciate ligament reconstruction.
Historically, BTB and doubled semitendinosus and gracilis HS grafts have been the most widely used and studied, but newer graft choices may offer increased benefits in the female athlete. With more recent methods of graft fixation, these graft types can be easily harvested and securely fixed in ACL tunnels.
A quadrupled semitendinosus graft is more robust when compared to a traditional 4-string doubled HS (Figure 1). The ability to have a graft that is reliably greater than 9 mm is essential to a successful ACLR, especially in a female athlete with smaller HS tendons. The quadriceps tendon is also advantageous as it is thicker compared to the patellar tendon. Similarly, this may be a better option in a female knee in which taking a greater than 9 mm patellar tendon graft may be difficult due to the size of the native tendon. Both a quadrupled semitendinosus and a quadriceps tendon graft are more robust in diameter compared to a patellar tendon.
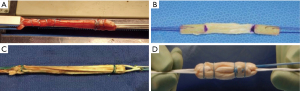
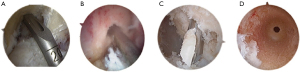
Female patients often have a smaller anatomical knee and smaller bone mass when compared to males resulting in a smaller notch, smaller tunnel lengths, and smaller autografts for harvest, and these factors should be considered when both preparing a graft and drilling the femoral and tibial tunnels. Current techniques allow for easier placement of anatomical tunnels and bone preservation. The use of an all-inside technique using retrograde tunnels with an independent femoral guide is potentially beneficial for females (Figure 2). This technique allows for the preservation of bone mass and has the decreased postoperative pain compared to other techniques (21). The use of an independent femoral guide allows ease of anatomic placement while avoiding potential risks of femoral wall blowout (23) and the ability to ensure a longer tunnel when compared to an anteromedial femoral drilling technique (Figure 2). The use of a graft with adequate diameter, greater than 9 mm, as well as a surgical technique that optimizes tunnel length and anatomic placement in females can hopefully lead to improved patient reported outcome measures (PROMs), RTS and decreased risk of re-tear (14).
RTS
Despite ACLR’s primary goal of restoring an individuals’ ability to RTS, the likelihood of incurring a secondary ACL injury post ACLR can reach ~50% (24-26). Lindanger et al. states that depending on the population in review, RTS to pre-injury level ranges between 40% and 80% (27). Paterno et al., showed that the incidence of second ACL injury in the first year following ACLR and RTS is 15 times greater compared to an uninjured cohort (28). Of the patients with a second ACL injury, ~17% sustained it to the contralateral knee and the mean time between RTS and the second ACL injury was 215 days. Further, this study showed that the rate of injury within the first 2 years of RTS for female athletes following ACLR was five times greater than female athletes without a history of ACL injury. Female athletes were also twice as likely to sustain a contralateral injury than an ipsilateral injury following ACLR (28). With this high incidence of second injuries in females, the criteria to allow female athletes to RTS after ACLR is increasingly important.
Ardern et al. investigated the RTS rate and participation level of a large cohort 1 year following ACLR (9). In this study, RTS was permitted at 9 months postoperatively with completion of full postoperative rehabilitation protocol, full range of motion (ROM), stable knee, functional quadriceps control, and no effusion. At the 1-year time point, only 33.4% of patients had returned to their pre-injury level of play with the other 66.6% either in training or had not yet attempted to train for their sport (9). This study also showed that females were significantly less likely than males to RTS in the first year, despite there being no difference in intention to RTS when compared with males. The results from Lindanger et al. add to this data as 83% of the athletes in their cohort returned to pivoting sports following ACLR, however only 53% returned to their pre-injury level. Their results did show RTS rates similar between males and females, but male’s career length was significantly longer than females who were also at higher risk of contralateral ACL injury (27).
If the goal of ACLR is to enable athletes to RTS at the same level prior to ACLR, then a postoperative protocol and RTS criteria that minimizes the risk of re-injury is essential. Since risk factors for ACL injury consist of sex, age, level of play, and prior or concomitant injuries, it is difficult to apply one set of criteria to all patients. Further, surgical technique, including graft diameter and placement, and rehabilitation can influence re-injury and are important to take into consideration (24,26). One review looking at current RTS criteria after ACLR, states that the term RTS must be accompanied by descriptive characteristics of the patients risk factors, use of protective equipment, and the type, level, and duration of play the athlete participates in (26).
The multifactorial RTS criteria includes time postoperatively, clinical examination, validated patient questionnaires, as well as psychological factors. Time is one of the most common criteria used for RTS. In a systematic review of 264 studies, 84 studies utilized postoperative time as the only criterion for RTS, 40 studies had amount of time combined with subjective criteria, and 35 studies included objective criteria such as general knee exams, muscle strength, single-leg hop tests, Lachman ratings, and validated questionnaires for RTS (25). Current literature varies, and the recommended duration of time from surgery to RTS ranges from 6 to 12 months and can even reach up to 2 years (7,26,28). For young athletes looking to play sports in college or beyond, a 2-year time frame, despite being potentially optimal for recovery, can be detrimental to their sports careers. This conflict is where other factors of RTS come into play.
Clinical examinations that measure muscle strength (both in operative and contralateral legs), hop tests to assess dynamic stability of the knee, and biomechanical deficits can help determine a patient’s readiness for sports. Muscle strength deficit, specifically in HSs and quadriceps, has been shown to increase the potential risk for future knee injuries following ACLR (26). Undheim et al. shows isokinetic dynamometry is a useful objective measurement for determining if a patient has adequate strength for RTS alongside limb symmetry index (LSI) scores that allow for quantitative comparisons between the index and contralateral leg (29). Isokinetic strength evaluation consists of a combination of concentric and eccentric knee extension and flexion (29). LSIs >85% to 90% are generally considered safe values, however this 15% difference may have a large impact on RTS readiness (26). This study does show however, that despite isokinetic tests, being used in RTS criteria, there is little standardization and recommendations range from greater than 80% to greater than 90% for the index knee when compared with the contralateral side (25). Another study, used the 90% or greater as passing RTS criteria for functional assessments (quadriceps strength index, single-hop test, crossover hop-test, triple-hop test, 6-meter time hop, and a global rating scale of overall knee function) along with Knee Outcome Survey-Activities of Daily Living Scale (KOS-ADLS), a validated questionnaire (30).
PROMs are often used to assess a patient’s perception of symptoms and function. Following ACL injury, PROMs such as the Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Outcome Survey-Sports Activity Scale (KOS-SAS), International Knee Documentation Committee 2000 Subjective Knee Form (IKDC-2000), Cincinnati Knee Score, Lysholm Score, Lower Extremity Functional Scale, and Tegner Activity Scale can be used to allow patients to detail their symptoms, ACL deficits, and overall knee deficits without clinician bias (31). Combining PROMs as well as objective performance based measurements are important when determining RTS readiness for athletes (32). Clinicians and athletes should use RTS criteria as guidelines for assessing whether an athlete can RTS and what level of play they can tolerate.
Psychological factors also play an important role in the level of sports participation following ACLR and are often overshadowed by functional measurements. Females have been shown to have greater levels of general anxiety postoperatively and demonstrate more emotionally oriented coping strategies, have greater stress reactions if they had high kinesiophobia, and compromised physical self-worth postoperatively (2). Following ACLR, females are often less likely to return to preinjury level of sport because of fear of re-injury rather than problems related to knee function (2). In a study by Ardern et al., preoperative and 4-month postoperative measurements of psychological readiness to RTS, fear of re-injury, locus of control, and athlete expectations successfully predicted the number of months it would take to return to the preinjury level of activity (greater number of months = less likely to actually RTS). Further, poor psychological responses on the Anterior Cruciate Ligament-Return to Sport after Injury scale (ACL-RSI) led to a lower RTS rate at preinjury level and worse functional outcomes (33). This study supported the idea that individuals are more likely to return to a sport or level of play where their competency levels are greater, and that psychological factors play a large role. For clinicians, addressing an athlete’s psychological readiness for RTS alongside the physical and functional components will maximize likelihood of a patient returning to preinjury level of play.
Conclusions
In summary, although the success rates of ACLR in female athletes is high, there is always room for improvement. Continuing to understand how to reduce graft failure rates and increase RTS rates in the female athlete after ACLR is critical. Consideration of graft choice and graft size is important in ACLR surgery, but even more important when treating female athletes where size of anatomy may require extra attention with preoperative planning. As surgical instruments and implants have been developed and upgraded, they can advantage us to improve these outcomes. Independent drill guides that may allow for easier anatomical placement and tunnel length adjustment may be important in smaller knees to guarantee adequate femoral tunnel length. Postoperative rehabilitation and RTS criterion are vital to getting female athletes back to activity safely (Table 2). Risk of re-tear, contralateral ACL injury, and decreased rates of RTS at the same level need to be ameliorated. Advancements have been made and more research is necessary to continue to optimize outcomes after ACLR in female athletes. Please find a supplemental Q&A between the authors and editors in Supplementary file (Appendix 1).
Table 2
| Consider anatomy |
| Size of knee, grafts available, sex-related differences |
| Ensure adequate graft measurements: |
| Size: >9 mm |
| Diameter: use of more robust quadrupled semitendinosus and quadriceps tendon grafts |
| Ensure adequate tunnel length and accurate anatomic placement |
| Consider use of independent femoral guide |
| Consider multifactor return to sport criteria |
| Time |
| Functional tests |
| PROMs |
| Psychological readiness |
ACLR, anterior cruciate ligament reconstruction; PROMs, patient reported outcome measures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sommer Hammoud and Robin V. West) for the series “Sports Related Injuries of the Female Athlete” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-31). The series “Sports Related Injuries of the Female Athlete” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salem HS, Varzhapetyan V, Patel N, et al. Anterior Cruciate Ligament Reconstruction in Young Female Athletes: Patellar Versus Hamstring Tendon Autografts. Am J Sports Med 2019;47:2086-92. [Crossref] [PubMed]
- Sims M, Mulcahey MK. Sex-Specific Differences in Psychological Response to Injury and Return to Sport Following ACL Reconstruction. JBJS Rev 2018;6:e9 [Crossref] [PubMed]
- Kaeding CC, Pedroza AD, Reinke EK, et al. Risk Factors and Predictors of Subsequent ACL Injury in Either Knee After ACL Reconstruction: Prospective Analysis of 2488 Primary ACL Reconstructions From the MOON Cohort. Am J Sports Med 2015;43:1583-90. [Crossref] [PubMed]
- Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med 2015;43:641-7. [Crossref] [PubMed]
- Davey AP, Vacek PM, Caldwell RA, et al. Risk Factors Associated With a Noncontact Anterior Cruciate Ligament Injury to the Contralateral Knee After Unilateral Anterior Cruciate Ligament Injury in High School and College Female Athletes: A Prospective Study. Am J Sports Med 2019;47:3347-55. [Crossref] [PubMed]
- Daruwalla JH, Greis PE, Hancock R, et al. Rates and Determinants of Return to Play After Anterior Cruciate Ligament Reconstruction in NCAA Division 1 College Football Athletes: A Study of the ACC, SEC, and PAC-12 Conferences. Orthop J Sports Med 2014;2:2325967114543901 [Crossref] [PubMed]
- Nagelli CV, Hewett TE. Should Return to Sport be Delayed Until 2 Years After Anterior Cruciate Ligament Reconstruction? Biological and Functional Considerations. Sports Med 2017;47:221-32. [Crossref] [PubMed]
- Ardern CL, Taylor NF, Feller JA, et al. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med 2012;40:41-8. [Crossref] [PubMed]
- Ardern CL, Webster KE, Taylor NF, et al. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med 2011;39:538-43. [Crossref] [PubMed]
- Poehling-Monaghan KL, Salem H, et al. Long-Term Outcomes in Anterior Cruciate Ligament Reconstruction: A Systematic Review of Patellar Tendon Versus Hamstring Autografts. Orthop J Sports Med 2017;5:2325967117709735 [Crossref] [PubMed]
- Björnsson H, Samuelsson K, Sundemo D, et al. A Randomized Controlled Trial With Mean 16-Year Follow-up Comparing Hamstring and Patellar Tendon Autografts in Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2016;44:2304-13. [Crossref] [PubMed]
- Haber DB, Brook EM, Whitlock K, et al. Predicting Quadrupled Graft Length and Diameter Using Single-Strand Tendon Dimensions in All-Inside Anterior Cruciate Ligament Reconstruction. Arthroscopy 2018;34:243-50. [Crossref] [PubMed]
- Park SY, Oh H, Park S, et al. Factors predicting hamstring tendon autograft diameters and resulting failure rates after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2013;21:1111-8. [Crossref] [PubMed]
- Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy 2013;29:1948-53. [Crossref] [PubMed]
- Conte EJ, Hyatt AE, Gatt CJ Jr, et al. Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy 2014;30:882-90. [Crossref] [PubMed]
- Nguyen D. Sex, Age, and Graft Size as Predictors of ACL Re-tear:A Multivariate Logistic Regression of a Cohort of 503 Athletes. Orthop J Sports Med 2016;4: 2325967116S00164.
- Magnussen RA, Lawrence JT, West RL, et al. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy 2012;28:526-31. [Crossref] [PubMed]
- Schurz M, Tiefenboeck TM, Winnisch M, et al. Clinical and Functional Outcome of All-Inside Anterior Cruciate Ligament Reconstruction at a Minimum of 2 Years' Follow-up. Arthroscopy 2016;32:332-7. [Crossref] [PubMed]
- Yasen SK, Borton ZM, Eyre-Brook AI, et al. Clinical outcomes of anatomic, all-inside, anterior cruciate ligament (ACL) reconstruction. Knee 2017;24:55-62. [Crossref] [PubMed]
- Kaeding CC, Pedroza AD, Reinke EK, et al. Change in Anterior Cruciate Ligament Graft Choice and Outcomes Over Time. Arthroscopy 2017;33:2007-14. [Crossref] [PubMed]
- Desai VS, Anderson GR, Wu IT, et al. Anterior Cruciate Ligament Reconstruction With Hamstring Autograft: A Matched Cohort Comparison of the All-Inside and Complete Tibial Tunnel Techniques. Orthop J Sports Med 2019;7:2325967118820297 [Crossref] [PubMed]
- Spragg L, Chen J, Mirzayan R, et al. The Effect of Autologous Hamstring Graft Diameter on the Likelihood for Revision of Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2016;44:1475-81. [Crossref] [PubMed]
- Osti M, Krawinkel A, Ostermann M, et al. Femoral and tibial graft tunnel parameters after transtibial, anteromedial portal, and outside-in single-bundle anterior cruciate ligament reconstruction. Am J Sports Med 2015;43:2250-8. [Crossref] [PubMed]
- Wiggins AJ, Grandhi RK, Schneider DK, et al. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am J Sports Med 2016;44:1861-76. [Crossref] [PubMed]
- Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy 2011;27:1697-705. [Crossref] [PubMed]
- Dingenen B, Gokeler A. Optimization of the Return-to-Sport Paradigm After Anterior Cruciate Ligament Reconstruction: A Critical Step Back to Move Forward. Sports Med 2017;47:1487-500. [Crossref] [PubMed]
- Lindanger L, Strand T, Molster AO, et al. Return to Play and Long-term Participation in Pivoting Sports After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2019;47:3339-46. [Crossref] [PubMed]
- Paterno MV, Rauh MJ, Schmitt LC, et al. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am J Sports Med 2014;42:1567-73. [Crossref] [PubMed]
- Undheim MB, Cosgrave C, King E, et al. Isokinetic muscle strength and readiness to return to sport following anterior cruciate ligament reconstruction: is there an association? A systematic review and a protocol recommendation. Br J Sports Med 2015;49:1305-10. [Crossref] [PubMed]
- Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2010;40:141-54. [Crossref] [PubMed]
- Lynch AD, Logerstedt DS, Grindem H, et al. Consensus criteria for defining 'successful outcome' after ACL injury and reconstruction: a Delaware-Oslo ACL cohort investigation. Br J Sports Med 2015;49:335-42. [Crossref] [PubMed]
- Logerstedt D, Arundale A, Lynch A, et al. A conceptual framework for a sports knee injury performance profile (SKIPP) and return to activity criteria (RTAC). Braz J Phys Ther 2015;19:340-59. [Crossref] [PubMed]
- Ardern CL, Taylor NF, Feller JA, et al. Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med 2013;41:1549-58. [Crossref] [PubMed]
Cite this article as: Matzkin EG, Lowenstein NA. Optimizing outcomes of anterior cruciate ligament (ACL) reconstruction in female athletes: from graft choice to return to sport criteria. Ann Joint 2021;6:40.


