
Hemiarthroplasty for proximal humerus fracture—a dying art
Introduction
Fractures of the proximal humerus are very common injuries, especially among elderly female patients. These fractures comprise 4–5% of all fractures of the appendicular skeleton (1) and are the third most common fracture in the geriatric population (2). Most commonly, these injuries occur as the result of low-energy trauma, like a ground level fall onto an outstretched arm (1,3,4). The incidence of these fractures has increased over the last three decades (4,5). The majority of proximal humerus fractures can be managed nonoperatively. For those fractures that do require surgery, the majority are treated with plate osteosynthesis or reverse shoulder arthroplasty (RSA). Despite the advances and increased utilization of these surgical techniques, hemiarthroplasty still has a role. Successful use of hemiarthroplasty for fracture relies on a clear understanding of anatomy, careful patient selection, surgical planning, and meticulous surgical technique.
Anatomy
The proximal humerus is composed of several distinct parts:
- Surgical neck—located at the proximal end of the diaphysis, at the metaphyseal flare and just distal to the tuberosities;
- Greater tuberosity—the attachment point for the posterosuperior rotator cuff tendons (supraspinatus, infraspinatus, teres minor), located at the superolateral margin of the articular surface;
- Bicipital groove—contains the long head of the biceps tendon and separates the greater tuberosity (posterolateral) from the lesser tuberosity (anteromedial);
- Lesser tuberosity—the attachment site for the subscapularis tendon;
- Anatomic neck—divides the articular surface of the humeral head from the tuberosities and the remainder of the metaphysis;
- Humeral head—the articular surface proximal to the tuberosities and articulates with the glenoid.
The articular surface of the humeral head is inclined an average of 130° relative to the humeral diaphysis and is retroverted an average of 20° relative to the trans-epicondylar axis of the distal humerus (4). The geometric center of the humeral head is offset at an average of 2.6 mm posteriorly and 7 mm medially to the center of the medullary canal, which is important for recreating normal kinematics during shoulder arthroplasty (4).
During fractures of the proximal humerus, displacement of fragments occurs due to the static and dynamic soft tissue attachments. The humeral shaft tends to displace medially, pulled by the latissimus dorsi and pectoralis major tendons. The greater tuberosity is pulled proximally, posteriorly and medially by the supraspinatus, infraspinatus and teres minor tendons. The lesser tuberosity is translated medially and anteriorly by the subscapularis tendon. Depending on the fracture pattern, the humeral head may displace along with one of the tuberosities, or it may be a free-floating fragment without tendinous attachments.
Understanding the vascular supply to the humeral head is also crucial in clinical decision-making. For some time, the consensus in the literature was that the anterior humeral circumflex artery (a branch of the axillary artery) is the primary source of blood supply to the humeral head (6,7). More recently, however, anatomic studies have disputed this thesis. Hettrich et al. used quantitative magnetic resonance angiography and gross dissection, to determine that the posterior circumflex humeral artery provides 64% of the perfusion to the humeral head (8). This posterior humeral circumflex predominance correlates with previous clinical observations that avascular necrosis (AVN) of the humeral head after proximal humerus fractures is relatively rare, despite the fact that the anterior humeral circumflex artery is disrupted more often than the posterior humeral circumflex. Furthermore, it has been observed that continuity between the posteromedial metaphysis (i.e., “the calcar”) and the articular surface on injury radiographs (which indicates that the posterior humeral circumflex artery is intact) is a protective factor against the development of AVN (9). Long-term outcomes of this hypothesis with a mean follow up of five years showed that, in a group of 10 humeral heads deemed “ischemic” by the above calcar measurement method, 80% did not develop AVN. This indicates that, despite arterial disruption at the time of injury, the humeral head may become reperfused over time in some patients (10).
Clinical evaluation
A patient with an acute proximal humerus fracture usually presents to the emergency department, most frequently after a low-energy ground-level fall, reporting severe shoulder pain and inability to move their arm. A careful neurovascular exam is critical. Because proximal humerus fractures are usually the result of ground-level falls in osteoporotic, elderly patients, one must evaluate for other fragility fractures, including hip and vertebral compression fractures.
The initial radiographic assessment of a suspected proximal humerus fracture consists of three mutually orthogonal radiographs, including a true anteroposterior (Grashey) view, a lateral (“scapular-Y”) view and an axillary view (4). This series allows the clinician both to characterize the fracture pattern and to identify a fracture-dislocation. If the patient cannot tolerate the arm abduction required to obtain a true axillary view, a Velpeau view may be obtained instead (11). Depending on the severity of displacement and comminution of the fracture, the clinician may decide to obtain a computerized tomography (CT) scan with 3-dimensional reconstructions. These studies may help the surgeon better understand the character of the fracture.
Fracture classification
The most commonly used classification system for proximal humerus fractures is that developed by Dr. Charles Neer in 1970, based on a series of 300 patients treated over a 14-year period (12). The system is based on the displacement of one or more of the four major segments of the proximal humerus as seen on orthogonal radiographs: (I) the humeral head; (II) the lesser tuberosity; (III) the greater tuberosity; and (IV) the humeral shaft. According to the modified classification system, a segment is considered to be a separate “part” only if it is displaced >1.0 cm or angulated >45°. Based on the number of displaced fragments, a given fracture can then be classified into one of four categories:
- One-part fractures: these are nondisplaced or minimally displaced fractures. These represent roughly half (49%) of all proximal humerus fractures (1).
- Two-part fractures: these involve displacement of the shaft, greater tuberosity, or lesser tuberosity. Two-part surgical neck fractures account for another quarter (28%) of all proximal humerus fractures (1).
- Three-part fractures: these involve separate displacement of any two segments relative to two remaining segments, which remain in continuity or minimally displaced. The most common combination is displacement of the greater tuberosity and the surgical neck, which account for roughly one tenth (9%) of all proximal humerus fractures (1).
- Four-part fractures: these are the least common but most severe fractures, accounting for only 3% of proximal humerus fractures (1).
Several investigators have found the interobserver reproducibility of the Neer system to be moderate at best, even when using CT scans in addition to plain radiographs (13,14). Several alternatives to the Neer classification have been proposed, but their use in clinical practice and the literature regarding proximal humerus fractures is limited (15).
Treatment
Treatment options for proximal humerus fractures include non-operative management, osteosynthesis with either plate or nail, and arthroplasty. Overall, there is significant controversy regarding indications for specific treatment modalities, largely because there is little high-quality evidence to guide decision-making.
Nonoperative treatment is by far the most common treatment strategy (3,5,16). By and large, this is the preferred treatment for one- and two-part proximal humerus fractures, which comprise the majority of fractures seen in clinical practice (1). After a short period of immobilization in a sling, the patient usually undergoes a rehabilitation program, beginning with pendulum exercises and progressing to passive range of motion, stretching, and strengthening (4).
Operative treatments can generally be divided into two categories: (I) osteosynthesis with preservation of the humeral head, and (II) humeral head-sacrificing arthroplasty. Minimally invasive techniques of closed reduction and percutaneous fixation have been described, but they are very rarely used today and are largely of historical interest (5,17). Contemporary locking plate technology was first introduced in 2002 and dramatically expanded the role of open reduction and internal fixation (ORIF) in management of these fractures, especially in elderly, osteoporotic bone (5). However, ORIF with proximal humeral locking plates has been shown to have a high complication rate (35%), and the reoperation rate after ORIF is as high as 25% (18). Major complications are twice as common in patients over the age of 60 than in younger patients.
There are two main arthroplasty options for proximal humerus fractures: hemiarthroplasty and RSA. Hemiarthroplasty involves replacing the humeral head with a stemmed prosthesis, with fixation of the tuberosities. This has been an option since Neer began using the implant for fracture in the 1950s. In the mid-2000s, surgeons began treating proximal humerus fractures with RSA (19). Since that time, the use of RSA for fracture has been accelerating, and there has been a concomitant decline in the use of hemiarthroplasty (5).
History of shoulder hemiarthroplasty for fracture
In the 1950s, surgeons began using prosthetic replacements made of acrylic, polyamide and polyethylene bearing surfaces in patients with acute unreconstructable proximal humerus fractures or sequelae of such fractures (20). In 1955, Neer reported the first results of vitallium (cobalt-chrome alloy) humeral prosthesis in a series of 12 patients with comminuted three- and four-part fractures (21,22). Hemiarthroplasty was proposed as a solution to the complications and poor outcomes associated with previous treatment options (21).
In a case series, published in 1970, Neer analyzed the functional outcomes of patients with proximal humerus fractures based on the fracture pattern and treatment used. He performed retrospective cohort study of 117 patients treated with closed reduction, ORIF or hemiarthroplasty. With ORIF, 100% of those with four-part fractures failed. With hemiarthroplasty, he observed satisfactory or excellent clinical outcomes in 96.9% and 73% for patients with three- and four-part fractures, respectively (17). Overall, he concluded that, although ORIF is a viable option for patients with three-part proximal humerus fractures, hemiarthroplasty provided superior results for patients with four-part fractures, which are at greater risk of developing AVN.
The design of the hemiarthroplasty prosthesis has evolved through three distinct generations in the time since Neer. The first generation Neer design consisted of the vitallium head with a stem machined with holes used to pass wires for fixation of the tuberosities. It was available in just three sizes: small, medium and large (22). In the 1990s, a second generation of humeral implant design was introduced to match the variation in head size and canal diameter observed in the population. However, the fixed geometry of these implants did not recreate normal anatomy, and their excessively large heads tended to “overstuff” the joint, which led to glenoid wear, instability, and over-tensioning of the rotator cuff tendons (22). The third generation of implants—in use today—was designed based on the observation that the center of the humeral head is offset posteriorly and medially relative to the humeral medullary canal. Therefore, today’s implants feature a modular, adaptable design that allows the surgeon to match each patient’s anatomy. Newer implants have instrumentation that optimizes use in fracture work, including jigs to dial in correct implant height and retroversion based on preoperative planning. The design of the prosthetic neck has also evolved to be more low-profile, facilitating anatomic reduction of the tuberosities (22).
Current use
Just as the overall treatment of proximal humerus fractures is variable, the use of hemiarthroplasty for fractures has varied geographically and over time (3). Kim et al. analyzed trends in shoulder arthroplasty in the USA from 2000 to 2008. They found that the incidence of hemiarthroplasty increased 1.5-fold (from 23 to 33 per 100,000 person per year) during this time. In 2008, 33% of hemiarthroplasties were performed for proximal humerus fractures—the second most common indication for the procedure behind osteoarthritis (43%) (23). More recently, McLean et al. analyzed national healthcare databases in Australia to investigate the changes in treatment patterns for proximal humerus fractures between 2008 and 2017. The incidence of proximal humerus fractures increased from 26.8 to 45.7 per 100,000 persons per year during this period (16). However, the overall operative management decreased from 32.5% to 22.8%, with hemiarthroplasty decreasing the most dramatically, from 19.3% of surgical treatments to just 3%. The authors attributed the overall decline in operative management to the impact of recent evidence [Proximal Fracture of the Humerus Evaluation by Randomization (PROFHER) trial] showing no functional differences between surgical and nonoperative treatments of displaced proximal humerus fractures of the surgical neck (24). The decline in hemiarthroplasty was attributed to the increasing use of RSA, which increased from 4.1% to 24.5% of all procedures (16).
Indications for hemiarthroplasty
Currently, the indications for hemiarthroplasty for proximal humerus fracture remain controversial, due in large part to the scarcity of high-quality evidence regarding outcomes of hemiarthroplasty versus ORIF and RSA. However, there is general consensus about the type of patient and fracture characteristics that may be amenable to hemiarthroplasty.
In order to be a candidate for hemiarthroplasty, a patient must first be medically stable and fit enough to undergo anesthesia and the physiologic stress of open surgery. Another factor to consider is the patient’s age. Elderly patients with osteoporotic bone and comminuted three- and four-part proximal humerus fractures—a population in which ORIF has shown high complication and reoperation rates—are ideal candidates for arthroplasty (25). Historically, this consisted of hemiarthroplasty. In recent years, however, RSA has supplanted hemiarthroplasty in the elderly population because the reverse prosthesis is less dependent on anatomic healing of the tuberosities and a functional rotator cuff.
In a younger population, however, hemiarthroplasty remains a good surgical option for carefully selected patients. Most young patients with proximal humerus fractures that require surgery can be treated successfully with ORIF, which preserves bone stock and restores anatomic alignment. However, if the articular surface has been injured beyond salvage, such as in a head-splitting fracture or fracture-dislocation, hemiarthroplasty may be the best option. As well, the surgeon may consider hemiarthroplasty for fracture in young patients with fracture patterns with high risk of ischemia and subsequent AVN. These include medial calcar extension <8 mm, disruption of the medial hinge, and four-part fracture-dislocations (26). Hemiarthroplasty is favorable in this young population (rather than RSA, as in the elderly population) because it preserves the glenoid, and because preinjury rotator cuff function is largely intact in this population. Additionally, younger patients typically have better bone quality and potential for healing the tuberosities.
Surgical technique
After induction of general anesthesia, the patient is placed on the operating room table in the beach chair position, with the head of bed elevated to 45° and the injured extremity free. The head of bed remains towards the anesthesiologist in order to accommodate the C-arm, which is brought in from the patient’s uninjured side. It is important to verify the ability to obtain orthogonal Grashey, scapular-Y and axillary lateral views with the C-arm before prepping and draping.
The operative arm is then placed into an articulating arm holder. Standard deltopectoral approach is performed. The biceps sheath is split sharply, and the long head of the biceps tendon is tenodesed to the upper border of the pectoralis major with two heavy nonabsorbable sutures in a figure-of-eight fashion. This has been shown to decrease long term pain and increase overall outcome measures (27).
Next, the fracture is inspected, and the main fragments identified. Irrigation is performed and fracture hematoma is removed. A Cobb elevator can help to liberate impacted fragments, especially in subacute fractures in which soft callus has formed. Multiple heavy nonabsorbable suture are then passed through the bone-tendon junction of each of the tuberosities using a modified Mason-Allen stitch. These sutures provide control over tuberosity position and are used to secure the tuberosities to the prosthesis later.
Attention is then turned to removal of the humeral head. If the head is a separate, displaced fragment, it is easily removed and capsular attachments are released with electrocautery. If the head is continuity with either tuberosity, it is removed with an oscillating saw or large osteotome, taking care to match the version and inclination of the native humeral head. The humeral canal is then identified and prepared with a canal finder and hand-held reamer. The appropriately-sized humeral prosthesis is then selected using the measured head size if able or using the glenoid size for initial estimation with subsequent trialing for congruity. Determining the depth at which to insert the implant depends somewhat on the fracture pattern. If the humeral calcar remains in continuity with the shaft, the prosthesis can be positioned just above the calcar. If the calcar is not in continuity with the shaft, then the stem should be inserted to a depth so that the top of the humeral head is 5.3–5.6 mm above the pectoralis major tendon, which has been shown to be a reliable landmark for recreating humeral height (28,29). Trialing can be difficult given the lack of bony support and control of trial height and rotation. We routinely use intra-operative fluoroscopy to confirm the position of the trial. Once the correct position and implant sizing is determined, we proceed to implanting the prosthesis. Humeral canal is irrigated, and a cement restrictor is placed. The canal is then filled with pressurized cement, and then the prosthesis is inserted into the cement mantle to the previously measured depth in 20 degrees of retroversion (30). This position is maintained until the cement cures. Alternatively, press-fitting the stem can be achieved if enough metaphyseal bone remains intact to obtain good axial and rotational stability. We then use the previously passed heavy nonabsorbable sutures to secure both the greater and lesser tuberosities to the prosthesis, taking care to reduce them into anatomic position. Biomechanical studies have shown that the addition of circumferential medial cerclage sutures to the prosthesis improves stability, decreases interfragmentary motion, and allows for earlier post-operative rehabilitation (31). After fully securing the tuberosities, we obtain Grashey, scapular-Y and axillary views of the proximal humerus to critically evaluate the position of the prosthesis and the tuberosities. Finally, the wound is irrigated and closed in standard fashion. Figure 1A,B,C,D,E and Figure 2A,B,C,D,E,F present imaging from two cases treated using the above operative treatment method.
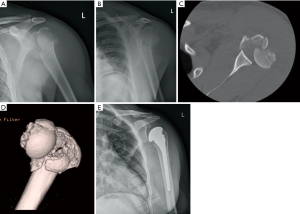
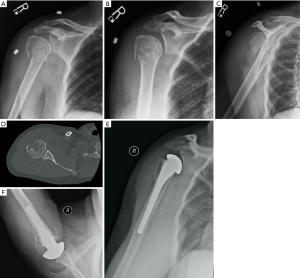
Outcomes
Primary hemiarthroplasty for proximal humerus fractures has been shown to have mixed results, but it can provide good pain relief and function. Restoration of humeral height, humeral version, and anatomic tuberosity reduction and healing are vital to promote excellent clinical outcomes after hemiarthroplasty.
Proper tuberosity positioning has been shown to correlate well with overall functional results. Frankle et al. demonstrated in a cadaveric study that anatomic reduction of the tuberosities around the prosthesis restored native shoulder kinematics (32). Alternatively, with malpositioning, an eightfold torque increase was required to achieve the same degree of external rotation. Radiographic malpositioning of the tuberosities has also been shown to correlate with poor functional results (33). Kralinger et al. showed that, tuberosity displacement >0.5 cm and tuberosity nonunion both correlate with worse Constant scores, patient satisfaction, and active forward elevation postoperatively (34). Overall, tuberosity nonunion is the most common cause of poor outcomes in hemiarthroplasty for proximal humerus fractures (35).
Humeral height and version are also important factors that correlate with outcomes. Boileau et al. showed that humeral height >10 mm compared to the contralateral, uninjured side produces increased supraspinatus tension and places increased stress on the tuberosity repair (33,36). Similarly, deviation from the anatomic retroversion of the humeral head (normally 19–22°) can also increase stress on the tuberosity repair. Boileau coined the term “the unhappy triad” of excessive height, retroversion, and greater tuberosity inferior malpositioning which lead to poor functional outcomes, persistent pain and stiffness (33).
Post-operative range of motion measures have been extensively reported via case series and systematic reviews with poor forward flexion (range, 101°–105.7°), external rotation 18°–30.4°, and mean abduction 92.4° (37,38).
Hemiarthroplasty for fracture has had mixed subjective and functional outcomes in the literature. Mighell et al. found in a retrospective review of 66 patients who underwent hemiarthroplasty for proximal humerus fracture that 93% were pain-free and satisfied with their overall result (38). Other studies paint a less satisfactory picture. Boileau et al. reported unsatisfactory outcomes in 42% of the patients treated with hemiarthroplasty (36). Boons et al. found no substantial difference in outcomes between shoulder hemiarthroplasty and non-operative treatment for four-part fractures (39). Kontakis et al. found a mean Constant score of 56.6 out of 100 in a study of 560 patients treated with hemiarthroplasty (37). Given these findings, hemiarthroplasty can be an adequate treatment option, but the surgeon must counsel patients about the low likelihood of returning to baseline shoulder function.
Complications
Shoulder hemiarthroplasty complications can generally be divided in early and late sequelae, with some overlap between groups. Major early complications include infection, intraoperative fracture, and damage to surrounding structures. Late complications include rotator cuff tear, infection, periprosthetic fracture, and aseptic loosening.
Infection
In a meta-analysis of 810 hemiarthroplasties, Kontakis et al. found the incidence of superficial and deep infections to be 1.55% and 0.64% respectively (37). Treatment of superficial infection typically involves returning to the operating room for thorough irrigation and debridement followed by culture-guided antibiotics. Management of deeper infection depends largely on the chronicity of infection. For acute deep infection, meticulous operative debridement with implant retention followed by antibiotics may suffice. Chronic deep infection necessitates implant removal, placement of antibiotic spacer, prolonged antibiosis, followed by delayed reimplantation.
Fracture
Although the rates of intraoperative fracture for primary shoulder arthroplasty have well documented (1.2%), there is limited data available for intraoperative humeral shaft fractures during arthroplasty for proximal humerus fractures (40). Based on surgeon preference, implant design, and patient factors, a cemented prosthesis may be used over a press-fit option in certain instances. Postoperative periprosthetic fracture is a rare complication (1.6–2.4%) that usually occurs due to additional trauma (41).
Nerve injury
Nerve injury is another potential complication. This may occur as a result of the initial trauma or because of dissection, retraction or manipulation of fracture fragments during surgery. The axillary nerve is the most commonly reported nerve injury, due to its location in the surgical field (42). Injury to the musculocutaneous nerve and other branches of the brachial plexus is also possible. These typically result from excessive traction. The majority of these nerve injuries are transient and resolve with observation and therapy. Another injury reported in the literature is iatrogenic radial nerve injury secondary to cement extrusion through the nutrient artery foramen of the humerus. Given this risk, we use a cement restrictor during cementation to prevent distal flow of the cement.
Tuberosity malunion/nonunion
Tuberosity malunion and nonunion remain the most important late complication that impairs overall outcome and function. In Boileau’s series of patient of 66, the author’s reported that malposition of the tuberosities occurred in 50% of patients, and this finding correlated with inferior functional outcomes (33). Malposition of the tuberosities can occur either as a result of malreduction at the time of surgery or from loss of reduction during the postoperative period. This underscores the importance of verifying tuberosity reduction intraoperatively both with direct visualization and with fluoroscopy. It is then equally important to ensure that the fixation construct is secure and rigid enough to maintain reduction until bony union.
Rotator cuff dysfunction can occur either in conjunction with—or independent of—tuberosity malunion/nonunion. Most patients undergoing hemiarthroplasty for fracture are at risk of cuff tears due to their age, history of trauma, and the placement of the humeral implant. If restoration of humeral height is not appropriately achieved and the joint is “overstuffed” (acromial humeral distance <2 mm) excess tension on the rotator cuff and subacromial impingement can lead to an attritional rupture (43). It can be very difficult to manage patients whose poor function after hemiarthroplasty is due to rotator cuff dysfunction or tuberosity issues. If tuberosity migration is detected early, it is possible to attempt revision fixation of the tuberosities. If malunion or nonunion is detected late, treatment involves either corrective osteotomies or revision to a reverse total shoulder arthroplasty.
Other complications
Stress shielding of the proximal humerus by the implant can lead to subsidence or aseptic loosening. Both can manifest as pain and loss of range of motion. Nagels et al. estimated the incidence of stress shielding to be approximately 9% (44). Glenoid erosion and radiographic osteoarthritis has also been reported to occur in up to 64% of patients (45). However, this seems to be well-tolerated and rarely requires revision.
Conclusions
Shoulder hemiarthroplasty for the treatment of proximal humerus fractures has long track record since the days of Neer. Once the primary treatment modality for fractures deemed unrepairable via osteosynthesis, hemiarthroplasty numbers have declined in recent years secondary to the introduction of RSA. Hemiarthroplasty is still an excellent treatment option in a very select patient population, specifically in young, active patients with fractures in which the humeral head is unreconstructable. Critical keys for successful outcomes include restoration of humeral height, humeral version, and most importantly anatomic tuberosity reduction and fixation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Management of Fractures Around the Shoulder”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-2019-mfas-08). The series “Management of Fractures Around the Shoulder” was commissioned by the editorial office without any funding or sponsorship. AJS served as the unpaid Guest Editor of the series. AJS reports personal fees from Medacta, personal fees from DJO, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Court-Brown CM, Garg A, Mcqueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand 2001;72:365-71. [Crossref] [PubMed]
- Beks RB, Ochen Y, Frima H, et al. Operative versus nonoperative treatment of proximal humeral fractures: a systematic review, meta-analysis, and comparison of observational studies and randomized controlled trials. J Shoulder Elbow Surg 2018;27:1526-34. [Crossref] [PubMed]
- Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am 2011;93:121-31. [Crossref] [PubMed]
- Streubel PN, Sanchez-Sotelo J, Steinmann S. Proximal Humeral Fractures. In: Tornetta P, Court-Brown CM, Heckman JD, et al. editors. Rockwood, Green, and Wilkins' Fractures in Adults. Philadelphia: LWW, 2014. 1341-426.
- Khatib O, Onyekwelu I, Zuckerman JD. The incidence of proximal humeral fractures in New York State from 1990 through 2010 with an emphasis on operative management in patients aged 65 years or older. J Shoulder Elbow Surg 2014;23:1356-62. [Crossref] [PubMed]
- Gerber C, Schneeberger AG, Vinh TS. The arterial vascularization of the humeral head. An anatomical study. J Bone Joint Surg Am 1990;72:1486-94. [Crossref] [PubMed]
- Brooks CH, Revell WJ, Heatley FW. Vascularity of the humeral head after proximal humeral fractures. An anatomical cadaver study. J Bone Joint Surg Br 1993;75:132-6. [Crossref] [PubMed]
- Hettrich CM, Boraiah S, Dyke JP, et al. Quantitative assessment of the vascularity of the proximal part of the humerus. J Bone Joint Surg Am 2010;92:943-8. [Crossref] [PubMed]
- Hertel R, Hempfing A, Stiehler M, et al. Predictors of humeral head ischemia after intracapsular fracture of the proximal humerus. J Shoulder Elbow Surg 2004;13:427-33. [Crossref] [PubMed]
- Bastian JD, Hertel R. Initial post-fracture humeral head ischemia does not predict development of necrosis. J Shoulder Elbow Surg 2008;17:2-8. [Crossref] [PubMed]
- Bloom MH, Obata WG. Diagnosis of posterior dislocation of the shoulder with use of Velpeau axillary and angle-up roentgenographic views. J Bone Joint Surg Am 1967;49:943-9. [Crossref] [PubMed]
- Neer CS 2nd. Displaced proximal humerus fractures. Part I. Classification and evaluation. J Bone Joint Surg Am 1970;52:1077-89. [Crossref] [PubMed]
- Sidor ML, Zuckerman JD, Lyon T, et al. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am 1993;75:1745-50. [Crossref] [PubMed]
- Bernstein J, Adler LM, Blank JE, et al. Evaluation of the Neer system of classification of proximal humeral fractures with computerized tomographic scans and plain radiographs. J Bone Joint Surg Am 1996;78:1371-5. [Crossref] [PubMed]
- Meinberg EG, Agel J, Roberts CS, et al. Fracture and Dislocation Classification Compendium-2018. J Orthop Trauma 2018;32:S1-S170. [Crossref] [PubMed]
- McLean AS, Price N, Graves S, et al. Nationwide trends in management of proximal humeral fractures: an analysis of 77,966 cases from 2008 to 2017. J Shoulder Elbow Surg 2019;28:2072-8. [Crossref] [PubMed]
- Neer CS. Displaced proximal humeral fractures. Part II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am 1970;52:1090-103. [Crossref] [PubMed]
- Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma 2009;23:163-72. [Crossref] [PubMed]
- Cazeneuve JF, Cristofari DJ. Grammont. Reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res 2014;100:93-7. [Crossref] [PubMed]
- Zilber S. Shoulder Prosthesis. Open Orthop J 2017;11:1099. [Crossref] [PubMed]
- Neer CS. Articular replacement for the humeral head. J Bone Joint Surg Am 1955;37-A:215-28. [Crossref] [PubMed]
- Boileau P, Sinnerton RJ, Chuinard C, et al. Arthroplasty of the shoulder. J Bone Joint Surg Br 2006;88:562-75. [Crossref] [PubMed]
- Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011;93:2249-54. [Crossref] [PubMed]
- Rangan A, Handoll H, Brealey S, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. JAMA 2015;313:1037-47. [Crossref] [PubMed]
- Chambers L, Dines JS, Lorich DG, et al. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskelet Med 2013;6:57-62. [Crossref] [PubMed]
- Boesmueller S, Wech M, Gregori M, et al. Risk factors for humeral head necrosis and non-union after plating in proximal humeral fractures. Injury 2016;47:350-5. [Crossref] [PubMed]
- Soliman OA, Koptan WM. Proximal humeral fractures treated with hemiarthroplasty: does tenodesis of the long head of the biceps improve results? Injury 2013;44:461-4. [Crossref] [PubMed]
- Murachovsky J, Ikemoto RY, Nascimento LG, et al. Pectoralis major tendon reference (PMT): a new method for accurate restoration of humeral length with hemiarthroplasty for fracture. J Shoulder Elbow Surg 2006;15:675-8. [Crossref] [PubMed]
- Sahu D, Jagiasi JD, Valavi AS, et al. The Distance between the Pectoralis Major Tendon Insertion and the Top of the Humeral Head is a Reliable Landmark: An Anatomic Study. Joints 2019;7:37-40. [Crossref] [PubMed]
- Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg 1998;7:144-6. [Crossref] [PubMed]
- Frankle MA, Ondrovic LE, Markee BA, et al. Stability of tuberosity reattachment in proximal humeral hemiarthroplasty. J Shoulder Elbow Surg 2002;11:413-20. [Crossref] [PubMed]
- Frankle MA, Greenwald DP, Markee BA, et al. Biomechanical effects of malposition of tuberosity fragments on the humeral prosthetic reconstruction for four-part proximal humerus fractures. J Shoulder Elbow Surg 2001;10:321-6. [Crossref] [PubMed]
- Boileau P, Krishnan SG, Tinsi L, et al. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 2002;11:401-12. [Crossref] [PubMed]
- Kralinger F, Schwaiger R, Wambacher M, et al. Outcome after primary hemiarthroplasty for fracture of the head of the humerus: a retrospective multicenter study of 167 patients. J Bone Joint Surg Br 2004;86:217-9. [Crossref] [PubMed]
- Bigliani LU, Flatow EL. Failed prosthetic replacement for displaced proximal humerus fractures. Orthop Trans 1991;15:747-8.
- Boileau P, Walch G, Krishnan SG. Tuberosity osteosynthesis and hemiarthroplasty for four-part fractures of the proximal humerus. Techniques in Shoulder and Elbow Surgery 2000;1:96-109. [Crossref]
- Kontakis G, Koutras C, Tosounidis T, et al. Early management of proximal humeral fractures with hemiarthroplasty: A systematic review. J Bone Joint Surg Br 2008;90:1407-13. [Crossref] [PubMed]
- Mighell MA, Kolm GP, Collinge CA, et al. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg 2003;12:569-77. [Crossref] [PubMed]
- Boons HW, Goosen JH, van Grinsven S, et al. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: A randomized controlled trial. Clin Orthop Relat Res 2012;470:3483-91. [Crossref] [PubMed]
- Athwal GS, Sperling JW, Rispoli DM, et al. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am 2009;91:594-603. [Crossref] [PubMed]
- Wright TW, Cofield RH. Humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 1995;77:1340-6. [Crossref] [PubMed]
- Plausinis D, Kwon YW, Zuckerman JD. Complications of humeral head replacement for proximal humeral fractures. Instr Course Lect 2005;54:371-80. [Crossref] [PubMed]
- Merolla G, Di Pietto F, Romano S, et al. Radiographic analysis of shoulder anatomical arthroplasty. Eur J Radiol 2008;68:159-69. [Crossref] [PubMed]
- Nagels J, Stokdijk M, Rozing PM. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg 2003;12:35-9. [Crossref] [PubMed]
- Wiater JM, Fabing MH. Shoulder arthroplasty: prosthetic options and indications. J Am Acad Orthop Surg 2009;17:415-25. [Crossref] [PubMed]
Cite this article as: Freeman TR, Dunn RH, Ko KJW, Seidl AJ. Hemiarthroplasty for proximal humerus fracture—a dying art. Ann Joint 2021;6:15.


