
Biofilm formation in periprosthetic joint infections
Introduction
Total joint arthroplasty has been one of the most successful procedures in the past few decades, resulting in substantial improvement in quality of life. However, periprosthetic joint infection (PJI) is a devastating potential complication with significant associated morbidity and mortality. While the overall incidence of PJI is relatively low, at approximately 1–2%, those affected experience significant loss of function and mobility (1). Additionally, associated healthcare costs can reach upward of $60,000 for a single episode (2). Even with resolution, the likelihood of developing a recurrent infection is significantly increased (3). As the frequency of joint arthroplasties continue to rapidly increase with an increasingly aging population, the need for new mechanisms of prevention and treatment is becoming urgent.
Diagnosis and treatment principles for PJI are remarkably challenging. Diagnosis of these infections is based upon a set of criteria, as no singular test is entirely sensitive and specific (4). Treatment principles are constantly evolving as the scientific community demonstrates a greater understanding of the mechanisms of pathogenesis. Approximately 65% of PJIs are thought to be related to the formation of bacterial biofilms in the joint space (5). A deeper understanding of this phenomenon will likely provide greater insight toward directing future diagnosis and treatment of these devastating infections.
Biofilm implication in PJIs
Most bacteria not only grow as free floating planktonic single cells, but rather as aggregates of cells in biofilms; this poses a significant clinical threat for many reasons (6). While planktonic bacteria may proliferate in body fluids and allow for seeding of distal sites, resulting in bacteremia and possibly sepsis (7) they are also relatively susceptible to host immune defenses and antibiotic therapy (8). In contrast, biofilm bacteria are limited in mobility and often tend to remain localized in an infection but have an arsenal of mechanisms of self-defenses: they coexist in a complex extracellular matrix that provides physical protection, allows the development of microenvironments, harbors slow growing and dormant sub-populations and facilitates intricate signaling between the cells within (9). The biofilm phenotype confers a characteristically high tolerance to antimicrobial challenge as well as resistance to host immune defenses, making clinical resolution of biofilm-associated infections uniquely taxing (8).
The majority of pathogens that cause PJIs have been shown to form biofilms to some extent (10) through laboratory experiments and microscopic examination of explanted materials. These bacteria include both gram-positive and gram-negative organisms, slow and fast growing, and aerobic or anaerobic (ESKAPE) pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species (11). The most common pathogens, Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa collectively make up approximately 75% of all identified organisms in PJI (11). Biofilms may also be multispecies in nature, or as tightly linked as independent aggregates.
The biofilm formation process may roughly be delineated into four steps (Figure 1) (12):
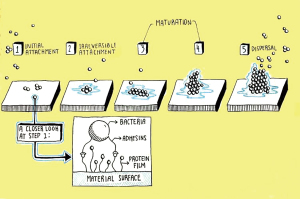
- Initial surface attachment of planktonic cells onto implant surfaces;
- Early biofilm formation-transition from the planktonic to biofilm phenotype with scant bacterial aggregates and the production of extracellular polymeric substances (EPS);
- Mature biofilm formation-consolidation of bacterial clusters into distinct microenvironments with unique pH and nutrient gradients, quorum sensing signaling, and secretion of virulence factors;
- Dispersal-release of select cells and aggregates into adjacent environment while the biofilm itself remains adherent to the substrate.
Biofilm establishment confers protection
Planktonic bacteria must overcome many challenges in order to survive long enough to form mature biofilms in the body. The process of survival, attachment, and subsequent proliferation requires mechanisms of self-preservation to be employed early on. Despite the heterogeneous nature of bacterial biofilms in respect to size, composition, and properties, there are consistent characteristics which contribute to increased resilience.
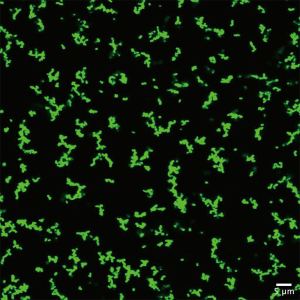
To initiate infection, bacterial cells must first bypass natural protective layers such as the dermis in order to seed at a favorable site (13). These bacteria that enter transcutaneously are believed to be in the planktonic state (13). Susceptible single cells must resist physical shear stress associated with synovial fluid flow in the joint, in addition to host immune defenses and antibiotic administration (14). It has recently been proposed that the ability to form bacterial aggregates upon entry into the joint cavity may confer protection from such volatile conditions (15). In the past few years, multiple groups have shown that exposure to synovial fluid stimulates the formation of macroscopic bacterial aggregates (Figure 2) (15,16). Early on, these synovial-fluid induced aggregates show recalcitrance to antibiotic challenge and neutrophil-mediated killing. Just 3 hours after exposure to synovial fluid, otherwise susceptible S. aureus as single cells when in aggregates display resistance to ×100 the minimum inhibitory concentration of Vancomycin (17).
Protection from host immune response
The presence of a bacterial invader, whether planktonic or biofilm, is expected to trigger the activation of the host immune system (18). Neutrophils will rapidly migrate to the site of infection and engage in phagocytosis, production of reactive oxygen species, and generation of neutrophil extracellular traps (NETs) to trap invading bacteria (19). These first responders are essential agents of infection control, and highly effective when combatting cells in a planktonic state (20). After the establishment of a mature biofilm has occurred, the physical size of the structure impedes the ability of the neutrophils to carry out successful phagocytosis (21). Recent findings suggest that conference of protection against phagocytic activity likely occurs early on in the process of biofilm formation. It has been shown that just 3 hours of seeded bacterial growth on a surface is sufficient to confer recalcitrance to neutrophil engulfment (22).
Another mechanism of protection employed by most bacterial invaders is the secretion of toxins, such as leucocidins (23). New evidence suggests that the production of these toxins could be advantageously stimulating leukocytes (which are unable to effectively phagocytose the biofilm aggregates due to their size and strength), to undergo extracellular trap formation (23). While originally thought of as an effective host defense, the formation of neutrophil extracellular traps (NETs) by extracellular DNA released by neutrophils, is now being investigated as a mechanism which may actually increase biofilm proliferation by providing additional biomaterial (23).
Adherence and proliferation
Should the bacteria survive these initial challenges, they may proceed to adhere to the periprosthetic tissue or implant surfaces themselves. There are a variety of mechanisms of bacterial adherence—one of the more well studied mechanisms in S. aureus species involves adhesins of the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) protein family (13,24). These proteins facilitate the binding of bacteria to an array of extracellular matrix proteins, including fibronectin (24). Adhesins also facilitate non-specific attachment to other orthopaedic components including polymers, metals, ceramics, sutures, and bone cement (25).
After adhesion, the planktonic bacteria undergo a replication phase resulting in proliferation and increasing colonization. The bacteria undergo a well-coordinated process in which specific genes are activated or deactivated in response to environmental stressors (26). Inter-bacterial communication, or quorum sensing, also occurs and regulates biofilm production (26). However, it is not known how long the proliferation process takes to initiate or the rate of growth in vivo. However, empirical evidence suggests that a quiescent biofilm may persist asymptomatically for months or years.
Producing an EPS matrix: creating a controlled microenvironment
Once attached, bacteria begin encapsulating themselves in a complex hydrogel EPS matrix predominantly composed of polysaccharides, glycoproteins, lipids, and extracellular DNA (9). These polymers interact uniquely through a variety of mechanisms including electrostatic interactions and cross-linking to develop interwoven complexes (27,28). The chemical composition of the EPS matrix can be highly variable, even between strains of the same bacteria, and it can change over time. In addition to bacterial produced polymers the biofilm EPS can incorporate host derived polymers. Bacteria in biofilms are fundamentally distinct phenotype from their planktonic counterparts, even though they share the same genotype. Certain bacterial cells in the biofilm may also undergo autolysis, resulting in free (extracellular) DNA that serves to maintain biofilm structural integrity (27). As the cells divide further with continued EPS formation, the biofilm structures coalesce together to form a more organized architecture.
Multi-dimensional layering of bacterial cells on a surface confers physical protection to bacteria residing within the interior of the biofilm. The complex EPS matrix creates a semi-enclosed microenvironment which acts as a protective barrier against both antimicrobials and the immune system defenses (29). As with any infection, the efficacy of an antimicrobial therapy is not based necessarily on completely eliminating the pathogen by itself, but to lower the burden enough to facilitate the hosts’ own immune system in clearing the infection. Depending on the composition and thickness of the biofilm, the diffusion of antimicrobials through a mature biofilm can be limited to the top layers (30). The ability of biofilms to tolerate high concentrations of antimicrobials, often orders of magnitude greater than that required to eradicate their planktonic counterparts, has been displayed across bacterial species and anatomical locations (8). During an immune response, the production of reactive oxygen species and other toxic mediators by leukocytes has little effect on the viability of an established biofilm (31).
Bacterial dormancy complicates therapeutic efficacy
It has been shown that diffusion through a mature biofilm is limited, which results in increasing nutritional and waste gradients as the biofilm grows (30). Bacteria on the periphery stay in a physiologic environment readily consuming glucose and oxygen, while cells in the interior become nutrient-deprived (32). Limited oxygen and nutrient availability, in conjunction with diffusion-limited buildup of metabolites and waste products, stimulate bacteria within the biofilm to enter a state of dormancy (32). A dormant bacterial cell will slow down processes deemed non-essential for its immediate survival, such as cell division and metabolic activity (33). This contributes to the lack of susceptibility to systemically tolerated levels of antimicrobials, most of which function by disrupting these processes.
The presence of persister cells within a biofilm community can complicate the resolution of an infection even further. These phenotypically distinct, highly resilient cells reside in a state of dormancy regardless of oxygen and/or nutrient availability. Although persisters only account for about 1% of the biofilm community, it has been shown that they cannot be killed by even high concentrations of antibiotics (8). During the administration of antibiotic therapy, these cells are essentially capable of “waiting out the storm” to eventually re-populate, often resulting in recurrent infections (34). Furthermore, persister cells still evoke a significant host inflammatory response that results in tissue destruction, osteolysis, and pain, as noted in chronic PJI (4). It is also thought that biofilms may persist insidiously for many months, even years, without overt clinical signs or symptoms of infection (35). Patients who had appeared asymptomatic for a considerable time after a previous revision for PJI, have been known to culture positive for the same infecting organism as prior to said revision.
Another survival mechanism that some bacterial species employ is capacity to develop resilient small-colony variant (SCV) phenotypes in biofilm populations. This genetic switch confers increased recalcitrance to antibiotic therapy as well as immune defenses (36). While the underlying molecular mechanisms of this switch are not entirely clear, recent works suggest that they are a manifestation of point mutations (37). Additionally, it has been shown that the number of SCVs present in a biofilm increase with the age of that biofilm and observed resilience (37). These highly resistant cells likely play a role in biofilm persistence and contribute to the frequency of recurrent infections.
Biofilm dispersal
As biofilms become nutrient starved, specific cell signaling pathways induce the production of hydrolases and surfactants that degrade the EPS polysaccharide pseudo capsule. Of note, the disassembly of the EPS matrix can vary between bacterial species. For example, Pseudomonas species initially dissolve the EPS polysaccharides via the release of hydrolases. Following this dissolution, newly released cells will transition into a planktonic phenotype expressing flagella used for motility out of the biofilm. In contrast, Staphylococcal species engage in two distinct phases during biofilm dispersal (38). The “exodus” phase of dispersal is characterized by nuclease production in order to degrade the early biofilm, which is predominantly composed of eDNA (38). The secondary phase of dispersal is characterized by protease production in order to degrade a matured biofilm, which is predominantly proteinaceous. Staphylococcal species are also known to produce phenol soluble modulins and detergents to further facilitate dispersion (38).
EPS degradation is essential for dispersal of planktonic bacteria or biofilm fragments into the local environment (27). It is unclear how dispersal translates into pathogenicity or clinical relevance. It is important to note that cells may be continually shed from biofilms at a background level, however a dispersal event occurs when many cells are released in a coordinated manner. Also, even though many cells may leave the biofilm in a dispersal event, many cells remain behind in the attached biofilm and PJI biofilm infections tend to remain localized in association with the implant surface. However, dissemination events may contribute to bacteremia and ultimately sepsis; furthermore, it may explain dissemination to other implants within the body (either a different prosthetic joint, or a device such as a pacemaker) (39).
Diagnostics
Both the Musculoskeletal Infection Society (MSIS) and the American Academy of Orthopaedic Surgeons have developed a united set of guidelines in diagnosing PJI, which are shown in the Table 1 (4). Often times these diagnostic criteria identify the presence of infection via proxy, since biofilm bacteria can be difficult to culture by standard clinical methods (40), and the criteria measure the host immune response to infection rather than directly distinguishing the pathogenic organism (41). However, in the absence of culture it can be difficult to distinguish an inflammatory response due to foreign body reaction from infection. Synovial fluid aspirate cultures, as well as intra-operative tissue cultures, are noted for a high rate of high negative results, especially as patients have often been put on a course of antibiotics prior to obtaining cultures (42). Alpha defensin, an antimicrobial peptide component of innate immunity thought only to be elevated in the presence of infection has shown promise as a diagnostic for PJI (4). Furthermore, bacteria from biofilm are inherently difficult to culture for diagnosis. As attached biofilms-they can be difficult to remove from the surface of tissue and implant materials (42). Sequencing molecular techniques, such as polymerase chain reaction, are currently being developed to detect bacterial ribosomal DNA and RNA (43). Still, these novel methodologies are imperfect as contaminant DNA can provide false positives. With the absence of culture, it is not possible to phenotypically verify an antibiogram against the infecting strain, or strains in the case of polymicrobial infections.
Table 1
| Periprosthetic joint infection diagnostic criteria | Diagnostic criteria |
|---|---|
| Major criteria: (at least one of following criteria) | (I) Two positive cultures of same organism; (II) sinus tract with communication to joint space or visualization of the prosthesis |
| Minor criteria: | 0–1: not infected; 2–5: inconclusive; ≥6: infected |
| Serum ESR | 1 |
| Serum CRP or D-Dimer | 2 |
| Synovial WBC or leukocyte esterase | 3 |
| Synovial alpha-defensin (+ result) | 3 |
| Elevated synovial PMN % | 2 |
| Elevated synovial CRP | 1 |
| Intraoperative diagnosis: Inconclusive pre-operative score (2 to 5) or dry tap | ≥6: infected; 4–5: inconclusive; ≤3: not infected |
| Pre-operative score | Inconclusive |
| Positive histology | 3 |
| Positive purulence | 3 |
| Single positive culture | 2 |
Adapted from Ref. (
Treatment
The effect of biofilms has significant implications with regard to the treatment of infection. There are two traditional paradigms in which PJI are grouped, which ultimately determine treatment: acute and chronic PJI (41). Broadly speaking, an acute infection occurs in one of two situations: either within 3–6 weeks of the index procedure or this “late acute PJI” may occur suddenly after the prosthesis has been functioning properly and is thought to be due to hematogenous seeding from a different site in the body. Late, or ‘chronic’ infection occurs more than 6 weeks beyond the initial surgery (or hematogenous seeding event). The timeline that differentiates an acute from chronic PJI is rather arbitrary and thus controversial. Acute infections are often initially treated with debridement, antibiotics, and implant retention (DAIR), consisting of irrigation and debridement along with a polyethylene exchange (44). Chronic infections are often treated with either a single-stage revision or a two-stage exchange (41).
The difference in management may be explained in the context of biofilm formation. Ostensibly, as long as the biofilm remains in the joint or on the implant, the infection will likely not be eradicated, and any surgical treatment that does not remove the affected portion of implant and tissue may ultimately be rendered futile. It is thought that with an acute infection, whether it be shortly (3–6 weeks) after the initial surgery, or seeding event in late acute PJI, the biofilm has not significantly established itself and thus a less involved procedure such as an irrigation and debridement with poly-exchange (DAIR) can be performed. However, in vitro studies show that biofilms can fully mature within just a matter of days (45). Thus, the time scales required to form a mature and protected biofilm is not well understood and largely assumed through anecdotal experience. Conversely with a chronic infection, the biofilm is thought to have fully matured in either the local tissue or the implant itself, and a more radical procedure is typically thought necessary to eradicate infection (41).
A single stage revision procedure generally involves irrigation and debridement with full removal of existing hardware, followed by immediate re-implantation of new hardware in the same procedure (46,47). A two-stage revision is characterized by a similar first stage step including irrigation and debridement with hardware removal. However, in lieu of a definitive implantation, an interim antibiotic cement spacer, formed from antibiotic-loaded methyl methacrylate bone cement (ALBC) and temporary components, can be placed (46,47). ALBC and bone graft substitutes such as calcium sulphate and calcium phosphate as antibiotic carriers have been used in treating PJI, as they can provide high local concentrations of the antibiotic unattainable by systemic administration (48). ALBCs can be used to cement implants definitively, or be placed temporarily, as in the form of a bead (bead chain) and spacer. ALBCs offer the distinct advantage of eluting antibiotics directly into the local joint space. However, the presence of antibiotics also compromises the mechanical strength of the cement, though this is less of a concern with beads and spacers, as they are meant to be provisional in nature. Furthermore, there are only certain antibiotics that are compatible with PMMA as the thermal curing process of PMMA can render heat-sensitive antibiotics non-functional. The efficacy of these carrier mediums is limited by surface-area and elution kinetics, as there is an initial burst release from the antibiotics located at the cements’ periphery (10). Antibiotic concentrations peak approximately three days after surgery, and sharply decline thereafter. Unfortunately, a substantial amount of antibiotics remain “locked” within the spacer as diffusion through PMMA is exceptionally slow. Calcium phosphate and sulfate do not have the mechanical strength of PMMA, but they are able to provide more consistent and prolonged release of antibiotics as they completely dissolve and release their antibiotic cargo (30) Furthermore, they also have a lower setting temperature than PMMA and are therefore compatible with many classes of antibiotics and antimicrobial agents (49). After the patient undergoes a prolonged (~6 weeks) course of antibiotics, they wait for a variable “cooling off period without antibiotics, to determine if infective symptoms and signs return, and then if no signs of infection are noted, undergo the second stage procedure with removal of the temporary antibiotic spacers and placement of definitive implants (46,47).
Both methods of treating chronic infection have merit and ultimately the same goal: to conclusively eradicate the infection by means of purging the biofilm in order to prevent reinfection. DAIR has been shown to have unacceptable failure rates in the treatment of chronic infection, presumably due to inability to fully expunge all remaining bacteria. As both single and two-stage procedures involve removal of implant components, and thus any potential biofilm present on their surface, as well as being able to access deeper osseous infected tissue around the removed components, this allows for better opportunity in removing all sites of potential infection. There is controversy surrounding whether a single-stage or a two-stage exchange is most appropriate. Intuitively, a two-stage exchange provides greater opportunities in eliminating biofilm bacteria as there are essentially two irrigation and debridement procedures, along with elution of local antibiotic loaded cement. The two-stage exchange is considered the gold standard of care in the United States, while data from Europe suggests equivocal outcomes with less morbidity in one-stage revisions.
Conclusions
The presence of biofilm in the joint space is now accepted as a serious clinical complication. Biofilm affects both diagnosis and treatment of PJI. Diagnosis of PJI remains a challenge in the absence of one singular testing modality with complete accuracy. Great efforts have been made by the MSIS (Musculoskeletal Infection Society), IDSA, and ICM (International Consensus Meeting) committees in recent years in optimizing diagnostic parameters for PJI. Nonetheless, biofilm formation decreases the yield from fluid or tissue culture, thus creating a significant obstacle to definitive microbial identification and treatment.
PJI treatment depends on several factors including the onset of symptoms, the specific pathogen suspected, and host health. Patients that present acutely (within 3–6 weeks) from the index procure or symptom onset are typically treated with DAIR, while chronic (>3–6 weeks) infections are treated with either a single-stage vs two-stage exchange arthroplasty. The treatment options are thought to differ because in the acute phase, biofilm has not sufficiently formed, and bacterial burden may be offloaded by a less invasive procedure. In contrast, a more comprehensive procedure is required to fully remove biofilm once it has already established itself in the local microenvironment.
In order to prevent and treat these devastating infections, it is imperative that the mechanism of biofilm establishment and subsequent immune response is understood. Most of the current methods for studying biofilm-associated joint infections are restricted to in vitro work, of which extrapolation to the clinic is limited. Over the past few years many groups in the field have introduced novel animal models in pursuit of replicating the complex patient system. In the future, a PJI-specific animal model will allow for a far more in-depth study of biofilm formation in the joint space. A thorough comprehension of the mechanism of biofilm formation at the microscopic level will better inform clinicians as to how to best combat them. Through this review the current understanding of establishment and proliferation in the joint space is discussed.
Acknowledgments
We thank Dr. Maurice Manring for compiling the manuscript for submission. We thank Ms. Sarah Davidson, UG student, Department of Art, OSU for compiling Figure 1.
Funding: This work was supported by the NIH grant R01GM124436 (to P Stoodley).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nemandra A. Sandiford, Massimo Francescini and Daniel Kendoff) for the series “Prosthetic Joint Infection” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-85). The series “Prosthetic Joint Infection” was commissioned by the editorial office without any funding or sponsorship. PS reports grants, personal fees and non-financial support from Biocomposites Ltd., personal fees from MicroGenDX, grants and personal fees from Smith and Nephew (wound care), personal fees from Zimmer-Biomet, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Springer BD, Cahue S, Etkin CD, et al. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today 2017;3:137-40. [Crossref] [PubMed]
- Boddapati V, Fu MC, Mayman DJ, et al. Revision Total Knee Arthroplasty for Periprosthetic Joint Infection Is Associated With Increased Postoperative Morbidity and Mortality Relative to Noninfectious Revisions. J Arthroplasty 2018;33:521-6. [Crossref] [PubMed]
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [Crossref] [PubMed]
- Goswami K, Parvizi J, Maxwell Courtney P. Current Recommendations for the Diagnosis of Acute and Chronic PJI for Hip and Knee—Cell Counts, Alpha-Defensin, Leukocyte Esterase, Next-generation Sequencing. Curr Rev Musculoskelet Med 2018;11:428-38. [Crossref] [PubMed]
- Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections: A review. Acta Orthop 2015;86:147-58. [Crossref] [PubMed]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol 2004;2:95-108. [Crossref] [PubMed]
- Peters BM, Jabra-Rizk MA, O’May GA, et al. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin Microbiol Rev 2012;25:193-213. [Crossref] [PubMed]
- McConoughey SJ, Howlin R, Granger J, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol 2014;9:987-1007. [Crossref] [PubMed]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol 2010;8:623-33. [Crossref] [PubMed]
- Masters EA, Trombetta RP, de Mesy Bentley KL, et al. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy.” Bone Res 2019;7:20. [Crossref] [PubMed]
- Santajit S, Indrawattana N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res Int 2016;2016:2475067 [Crossref] [PubMed]
- Coughlin MJ. Bacterial Biofilms and Periprosthetic Infections. J Bone Jt Surg 1996;78:932-66. [Crossref]
- Darouiche RO. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clin Infect Dis 2001;33:1567-72. [Crossref] [PubMed]
- Wang P, Guan PP, Guo C, et al. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J 2013;27:4664-77. [Crossref] [PubMed]
- Pestrak MJ, Gupta TT, Dusane DH, et al. Investigation of synovial fluid induced Staphylococcus aureus aggregate development and its impact on surface attachment and biofilm formation. PLoS One 2020;15:e0231791 [Crossref] [PubMed]
- Dastgheyb S, Parvizi J, Shapiro IM, et al. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis 2015;211:641-50. [Crossref] [PubMed]
- Gilbertie JM, Schnabel LV, Hickok NJ, et al. Equine or porcine synovial fluid as a novel ex vivo model for the study of bacterial free-floating biofilms that form in human joint infections. PLoS One 2019;14:e0221012 [Crossref] [PubMed]
- de Vor L, Rooijakkers SHM, van Strijp JAG. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett 2020;594:2556-69. [Crossref] [PubMed]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159-75. [Crossref] [PubMed]
- Papayannopoulos V. Neutrophils Facing Biofilms: The Battle of the Barriers. Cell Host Microbe 2019;25:477-9. [Crossref] [PubMed]
- Scherr TD, Heim CE, Morrison JM, et al. Hiding in plain sight: Interplay between staphylococcal biofilms and host immunity. Front Immunol 2014;5:37. [Crossref] [PubMed]
- Ghimire N, Pettygrove BA, Pallister KB, et al. Direct microscopic observation of human neutrophil-Staphylococcus aureus interaction in vitro suggests a potential mechanism for initiation of biofilm infection on an implanted medical device. Infect Immun 2019;87:e00745-19. [PubMed]
- Gutierrez Jauregui R, Fleige H, Bubke A, et al. IL-1β Promotes Staphylococcus aureus Biofilms on Implants in vivo. Front Immunol 2019;10:1082. [Crossref] [PubMed]
- Foster TJ. The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol 2019;27:927-41. [Crossref] [PubMed]
- Khatoon Z, McTiernan CD, Suuronen EJ, et al. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018;4:e01067 [Crossref] [PubMed]
- Subhadra B, Kim DH, Woo K, et al. Control of biofilm formation in healthcare: Recent advances exploiting quorum-sensing interference strategies and multidrug efflux pump inhibitors. Materials (Basel) 2018;11:1676. [Crossref] [PubMed]
- Mann EE, Rice KC, Boles BR, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 2009;4:e5822 [Crossref] [PubMed]
- Kavanaugh JS, Flack CE, Lister J, et al. Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio 2019;10:e01137-19. [Crossref] [PubMed]
- Flemming HC, Wingender J, Szewzyk U, et al. Biofilms: An emergent form of bacterial life. Nat Rev Microbiol 2016;14:563-75. [Crossref] [PubMed]
- Doroshenko N, Tseng BS, Howlin RP, et al. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob Agents Chemother 2014;58:7273-82. [Crossref] [PubMed]
- Yamada KJ, Kielian T. Biofilm-Leukocyte Cross-Talk: Impact on Immune Polarization and Immunometabolism. J Innate Immun 2019;11:280-8. [Crossref] [PubMed]
- Wan N, Wang H, Ng CK, et al. Bacterial metabolism during biofilm growth investigated by13C tracing. Front Microbiol 2018;9:2657. [Crossref] [PubMed]
- Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Appl Environ Microbiol 2013;79:7116-21. [Crossref] [PubMed]
- Molina-Manso D, Del Prado G, Ortiz-Pérez A, et al. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int J Antimicrob Agents 2013;41:521-3. [Crossref] [PubMed]
- Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat Rev Microbiol 2017;15:453-64. [Crossref] [PubMed]
- Dusane DH, Brooks JR, Sindeldecker D, et al. Complete killing of agar lawn biofilms by systematic spacing of antibiotic-loaded calcium sulfate beads. Materials (Basel) 2019;12:4052. [Crossref] [PubMed]
- Kittinger C, Toplitsch D, Folli B, et al. Phenotypic stability of staphylococcus aureus small colony variants (Scv) isolates from cystic fibrosis (cf) patients. Int J Environ Res Public Health 2019;16:1940. [Crossref] [PubMed]
- Paharik AE, Horswill AR. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol Spectr 2016;4: [Crossref] [PubMed]
- Barrett L, Atkins B. The clinical presentation of prosthetic joint infection. J Antimicrob Chemother 2014;69:i25-7. [Crossref] [PubMed]
- Saeed K, McLaren AC, Schwarz EM, et al. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. J Orthop Res 2019;37:1007-17. [Crossref] [PubMed]
- Osmon DR, Berbari EF, Berendt AR, et al. Executive Summary: Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1-10. [Crossref] [PubMed]
- Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007;357:654-63. [Crossref] [PubMed]
- Cazanave C, Greenwood-Quaintance KE, Hanssen AD, et al. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J Clin Microbiol 2013;51:2280-7. [Crossref] [PubMed]
- Qasim SN, Swann A, Ashford R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement - a literature review. SICOT J 2017;3:2. [Crossref] [PubMed]
- Lebeaux D, Chauhan A, Rendueles O, et al. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013;2:288-356. [Crossref] [PubMed]
- Kaufman MG, Meaike JD, Izaddoost SA. Orthopedic Prosthetic Infections: Diagnosis and Orthopedic Salvage. Semin Plast Surg 2016;30:66-72. [Crossref] [PubMed]
- Charette RS, Melnic CM. Two-Stage Revision Arthroplasty for the Treatment of Prosthetic Joint Infection. Curr Rev Musculoskelet Med 2018;11:332-40. [Crossref] [PubMed]
- Urish KL, DeMuth PW, Kwan BW, et al. Antibiotic-tolerant Staphylococcus aureus Biofilm Persists on Arthroplasty Materials. Clin Orthop Relat Res 2016;474:1649-56. [Crossref] [PubMed]
- Laycock PA, Cooper JJ, Howlin RP, et al. In vitro efficacy of antibiotics released from calcium sulfate bone void filler beads. Materials (Basel) 2018;11:2265. [Crossref] [PubMed]
Cite this article as: Staats A, Li D, Sullivan AC, Stoodley P. Biofilm formation in periprosthetic joint infections. Ann Joint 2021;6:43.


