The ‘2-in-1’ stage: indications, technique & results
Introduction
Primary total knee arthroplasty (TKA) is a successful surgical treatment for end-stage arthritis of the knee, with over 1,000,000 procedures having been carried out in the United Kingdom over the last 20 years (1). Approximately 97% are carried out for osteoarthritis, with more women than men undergoing the procedure and mean age at the time of surgery of 68.7 years (1). In the United States it is estimated that over the next 30 years there will be 673% increase in demand for TKA and consequently a likely increase in revision burden (2). As with many surgical procedures there is a small, but significant, complication rate with peri-prosthetic deep infection posing a serious challenge for both patient and surgeon. Infection as a complication post primary TKA has an incidence of between 0.5–2% following TKA (3,4). Deep infection accounts for ~25% of all revision TKA, in 2014 there were 1600 rTKA carried out for deep infection in the United Kingdom (NJR), and in USA 22,000 carried out in 2009 (2). rTKA creates an economic burden and reports from the UK have estimated the cost being in the region £75,000 per patient (5) and those patients are likely have a hospital stay twice that and three times the cost of a rTKA for aseptic loosening (6).
The risk of infection post TKA is increased in patients with rheumatoid arthritis, previous joint surgery and a higher Charleston co-morbidity index (7,8). Other studies have suggested increased risks with a surgical site infection not involving a prosthetic joint, National Nosocomial Infections Surveillance (NNIS) systems surgical patient risk index of 1 or 2 or an existing malignancy (9).
For patients with a deep infection post TKA, there are three potential treatment options: debridement irrigation and implant retention (DAIR), 1 stage revision arthroplasty and 2 stage revision arthroplasty. DAIR has a specific, but limited, role in treating deep infection with success rates varying between 14–100% (10,11). It works optimally in healthy patients presenting acutely following onset of symptoms with a sensitive organism and well fixed implants. Two stage revision refers to the removal of all implants, debridement of surrounding tissues, insertion of an antibiotic delivery system and temporary spacer implanted followed by a period of systemic antibiotic treatment and then re-implantation. Single stage revision refers to debridement and exchange of implants during the same procedure.
Two stage revision has been considered the ‘Gold Standard’ for treatment of PJI in TKA following its description by Insall in 1983 (12). The original procedure has been modified with the introduction of first static spacers (13) then more recently articulating spacers. There are very good reasons for its success rate and ongoing use, with 2 opportunities to debride the tissues and time to assess the success of antimicrobial treatment. There are drawbacks to the procedure as it imposes a heavy burden on the patient and health care system. The variable interval period can debilitate a patient, the spacer used may create complications (6) and there are risks to the patient from undergoing 2 major procedures. All of which contributes to a significant financial impact from this treatment option. Unsurprisingly this has led to repeated interest in the option of single stage revision as an alternative. Results from some European centres have shown success rates similar to two stage procedures when used in appropriately selected patients with thorough surgical approach and technique (14,15). This article describes the use of a variation of single stage revision, a ‘2-in-1’ technique, and outcomes from its use in PJI.
Indications
Single stage rTKA is not a new concept having been described by Bucholtz in the 1970’s with subsequent reports by Borden & Gearen in 1987 (16) and Göksan & Freeman in 1992 (17). Increasingly there has been interest in this technique as an alternative treatment in delayed deep prosthetic infection post TKA (14) with the advantages of a single procedure, shorter antibiotic period and reduced costs whilst achieving comparable success rates. The ‘2-in-1’ approach is a variation of this, allowing for 2 distinct, separate stages to be carried out under a single anaesthetic and patient admission but with the interval between the stages shortened to around 20 minutes.
The exclusion criteria used for this approach is (18):
- Failure >2 previous one stage procedures;
- Infection spreading to the neurovascular bundle;
- Unclear pre-operative bacterial specification;
- Antibiotics required have poor or variable bioavailability;
- Infecting bacteria have high or multiple resistance to antibiotics;
- Complex sinus or one distant to the proposed incision.
Technique
The indication for using this particular technique in revision of infected TKA is a proven deep infection defined by MSIS criteria (19). Infection was diagnosed by raised inflammatory markers (FBC, CRP & ESR/PV), positive joint aspirate or same bacterial growth on multiple deep tissue samples (same organism on >3 samples).
The procedure can be carried out under either general anaesthesia, spinal or epidural anaesthesia or a combination of both. Pre-operatively all cases should be discussed with a consultant microbiologist, with a special interest in musculoskeletal infection, and ideally via a multi-disciplinary team. A tailored antibiotic protocol should be drawn up individually for each case. The procedure is carried out with the use of a thigh tourniquet with the incision made through the most lateral previous scar using a medial parapatellar approach. The original scar is excised along with any sinus present and the current prosthesis is explanted. With a ‘2-in-1’ variation, the procedure is split into 2 stages which distinctly separates the debridement from reconstruction. In the first part of the procedure, the debridement should be split into: Implant removal, soft tissue debridement, bone debridement and thorough irrigation.
Where access is difficult, a tibial crest osteotomy (20) or an extensile medial parapatellar approach (21) may assist access. Initially fluid samples are taken plus at least 6 tissue samples, obtained with separate instruments, from different areas of the knee are taken to be sent for culture and sensitivity. Explantation of the existing implants should be carried out with a combination of punches, osteotomes (flexible or rigid) and oscillating saw with the aim of extracting them with as minimal bone loss as possible prior to commencing the debridement.
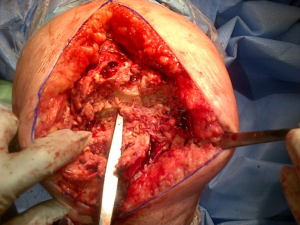
A radical debridement should be carried out in 2 stages, initially of the soft tissues and synovium of the knee, back to healthy tissue. Once this is complete excision of any infected, necrotic or devitalized bone should be carried out and followed by a thorough irrigation via pulsatile lavage with a minimum of 9 liters of normal saline and the intra-medullary canals packed with gauze swabs soaked with chlorhexidine. The irrigation may be augmented dilute hydrogen peroxide, aqueous chlorhexidine or antibiotics as each individual case requires. Once complete, the wound should be loosely closed and the wound circumferentially bandaged prior to deflating the tourniquet. All patients should receive 1g tranexamic acid intravenously and the tourniquet can be deflated. This completes the first part of the procedure (the debridement, Figure 1) and is equivalent to the first stage in a standard two-stage. At this point all existing operative kit should be discarded and the whole surgical team should re-scrub. Ideally an interval of approximately 20 minutes gives time for the theatre to be cleaned, new sterile operative instruments and kit to be re-introduced for the next (sequential second stage).
The tourniquet is re-inflated and the leg prepped and covered with sterile drapes. Once any packing gauze has been removed, any residual femoral or tibial bone-loss in each case is then assessed by the primary surgeon using the Anderson Orthopaedic Research Institute (AORI) grading. Following grading, a decision is made regarding the most appropriate construct accounting for any bone loss or damage to the collateral ligaments. The femoral component is sized, and augments and stems are used if needed. Any peripheral or cavitatory defects can be addressed with augments, metaphyseal sleeves or cones and the same approach is employed in the tibia. The use of metaphyseal sleeves for revision knee arthroplasty, particularly in cases involving bone loss, have reported good short and medium term results. Their use has been reported to be as effective in setting of infected knee arthroplasty particularly provide stable fixation of implants and contributing to the overall debridement (22,23). Depending on the degree of bone and soft tissue debridement, then an increase in the level of constraint may be necessary for a stable prosthesis construct. The components are cemented in place with additional antibiotics in the cement (Gentamicin loaded Palacos cement) as advised. At the end of the procedure, a second 500 mg of tranexamic acid was given. Suction drains are not required routinely in these cases.
Patients should be nursed post operatively on a specialist orthopaedic ward with a mobilisation protocol allowing full weight bearing immediately. All patients should receive post-operative anticoagulation therapy based on an individualized risk assessment for venous thromboembolism, antibiotics are commenced immediately post-op and adjusted according to final microbiological results of fluid, bone and soft tissue samples. The timing of conversion to oral antibiotics is decided by microbiology advice on a case by case basis. This duration of the oral therapy may vary from 4 weeks to 6 months. Most patients require treatment with two oral agents, which are usually continued for at least 4 weeks after the CRP returns to normal. Serial CRP measurements can be used to monitor the response to post-surgery antibiotic therapy.
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Results
Where it is safe to do so, a single stage procedure can avoid several of the drawbacks which may occur with a formal two stage approach. Particularly, it can reduce the risk of post-operative stiffness and arthrofibrosis which can be associated with two stage surgery. Use of a single stage be more cost effective by saving the patient having to undergo a second major procedure. These, coupled with a success rate very comparable to two-stage surgery, may become a potentially more attractive option for the patient and health care provider.
To date there are no prospective randomised controlled trials directly comparing one stage with two stage revision for deep infection post TKA. There are recent systematic reviews and meta-analysis (18,24,25) reporting published results for single stage to be between 86–100% and 66–95% for two stage procedures. It is important to note that patients undergoing one-stage revisions are likely to be pre-selected on the basis of the MSIS criteria (19) and many two-stage procedures may not be suitable for a single stage procedure. This is likely to affect the results of two stage and should be considered when comparing the results.
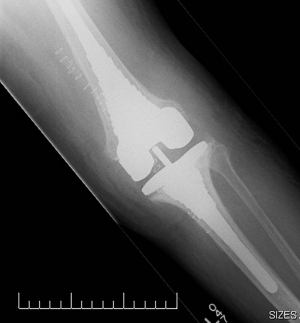
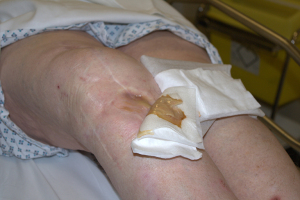
There are a small number of reports detailing ‘2-in-1’ stage procedures, with a series from Parkinson et al. (22) describing 12 cases managed using this method with no cases reporting recurrent infection at a minimum of 2 years post procedure. It is of interest that 2 cases included in this series had an actively discharging sinus pre-operatively, which is commonly cited as a contra-indication to one-stage revision. Although this is a small series and caution should be exercised in extrapolating the results, it raises the point that a patient with deep infection and a solitary sinus, may be safely treated with a single stage procedure if the sinus can be fully excised along with the initial debridement. A further study reported a series of 25 patients (26) followed up to between 2–8.5 years reported an eradication rate of 96%. This series involved the use of uncemented metaphyseal sleeves in the reconstruction of significant bone defects associated with deep infection (Figure 2). Similar to the preceding study, this one included six patients with a draining sinus pre-operatively (Figure 3), although all were through or adjacent to the original incision. The successful treatment of these via this method, lends support to this specific criterion not being an absolute contra-indication to a single stage procedure. While there is no long term data for this approach, results from Zahar et al., reporting on 46 patients undergoing a single stage procedure having an infection free survival of 93% at 10 years post procedure, despite not adhering to a strict selection criteria protocol. Further long term results will be required to support and establish the exact place for this technique within single stage surgery for PJI. It also highlights the need for ongoing work to further define the role of selection criteria when deciding the suitability of a patient for single stage surgery.
The function of the final construct is also important to the patient. Kunutsor et al. compared both one and two stage procedures and recorded Knee Society Scores of 80.3 and 82.1 respectively. The series by Holland et al. (26) noted improvement in KSS scores mirroring this and also the average Oxford knee score of 36.1 which is directly comparable to both conventional single and two stage procedures. This is particularly marked when the pain component scores are examined, showing a significant improvement. Lastly, there is also a significant financial consideration for both patient and provider in that successful treatment can be carried out from a single hospital stay.
Conclusions
Peri-prosthetic joint infection continues to be devastating complication for the patient and with the current literature unable to either demonstrate superiority of one or two stage revision then we should continue to assess on a case by case basis. Ideally large randomised controlled, comparative trials are needed to guide which approach benefits patients optimally. As detailed here, the ‘2-in-1’ approach for appropriately selected patients offers an alternative option for managing these patients successfully. The key features of this procedure are summarised in Table 1.
Table 1
| Pre-operative identification of the infecting organism. Take multiple samples (min 6) for culture and sensitivity |
| 2 separate components to the procedure: debridement (soft tissue & bone plus irrigation) and reconstruction |
| Break between stages of ~20 mins, wound closed, theatre cleaned, staff re-scrub and new sterile instruments |
| Commence IV antibiotics immediately post procedure guided by microbiology and continue for a minimum of 4–6 weeks |
| Adjust antibiotics according to intra-operative sample results |
| Allow immediate weight-bearing post operatively along with range of movement exercises |
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nemandra A Sandiford, Massimo Francescini and Daniel Kendoff) for the series “Prosthetic Joint Infection” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-83). The series “Prosthetic Joint Infection” was commissioned by the editorial office without any funding or sponsorship. PJW reports personal fees from DePuy J&J, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Registry, National Joint. Available online: https://www.hqip.org.uk/resource/national-joint-registry-15th-annual-report-2018/#.XqMEpeTsY2w. 2018.
- Kurtz S, Mowat F, Ong K, et al. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am 2005;87:1487-97. [PubMed]
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br 2004;86:688-91. [Crossref] [PubMed]
- Lentino JR. Infections Associated with Prosthetic Knee and Prosthetic Hip. Curr Infect Dis Rep 2004;6:388-92. [Crossref] [PubMed]
- Oduwole KO, Malony DC, Walls RJ, et al. Increasing financial burden of revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2010;18:945-8. [Crossref] [PubMed]
- Kallala RF, Vanhegan IS, Ibrahim MS, et al. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J 2015;97-B:197-201. [Crossref] [PubMed]
- Mortazavi SM, Schwartzenberger J, Austin MS, et al. Revision total knee arthroplasty infection: incidence and predictors. Clin Orthop Relat Res 2010;468:2052-9. [Crossref] [PubMed]
- Fehring KA, Abdel MP, Ollivier M, et al. Repeat Two-stage Exchange Arthroplasty for periprosthetic knee infection is dependent on host grade. J Bone Joint Surg Am 2017;99:19-24. [Crossref] [PubMed]
- Pan A, Cauda R, Concia E, et al. Consensus document on controversial issues in the treatment of complicated skin and skin-structure infections. Int J Infect Dis 2010;14:S39-53. [Crossref] [PubMed]
- Vasso M, Schiavone Panni A. Low-grade periprosthetic knee infection: diagnosis and management. J Orthop Traumatol 2015;16:1-7. [Crossref] [PubMed]
- Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 2009;63:1264-71. [Crossref] [PubMed]
- Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. JBJS Am 1983;65:1087. [Crossref] [PubMed]
- Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res 1997;148-54. [Crossref] [PubMed]
- Gehrke T, Zahar A, Kendoff D. One-stage exchange: it all began here. Bone Joint J 2013;95-B:77-83. [Crossref] [PubMed]
- Gulhane S, Vanhegan IS, Haddad FS. Single stage revision: regaining momentum. JBJS Br 2012;94:120-2. [PubMed]
- Borden LS, Gearen PF. Infected total knee arthroplasty. A protocol for management. J Arthroplasty 1987;2:27-36. [Crossref] [PubMed]
- Göksan SB, Freeman MA. One-stage reimplantation for infected total knee arthroplasty. J Bone Joint Surg Br 1992;74:78-82. [Crossref] [PubMed]
- Kunutsor SK, Whitehouse MR, Blom AW, et al. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0150866. [Crossref] [PubMed]
- Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992-4. [Crossref] [PubMed]
- Agarwal S, Azam A, Morgan-Jones R. Metal metaphyseal sleeves in revision total knee replacement. Bone Joint J 2013;95-B:1640-4. [Crossref] [PubMed]
- Stevens JM, Clement ND, Macpherson G, et al. Comparison of two extensile approaches to the knee: a cadaveric study evaluating quadriceps snip and extensile medial parapatellar approach. J Orthop 2018;15:416-9. [Crossref] [PubMed]
- Parkinson RW, Kay PR, Rawal A. A case for one-stage revision in infected total knee arthroplasty? Knee 2011;18:1-4. [Crossref] [PubMed]
- Klim SM, Amstofer F, Bernhardt GA, et al. Septic Revision Total Knee Arthroplasty: Treatment of Metaphyseal Bone Defects Using Metaphyseal Sleeves. J Arthroplasty 2018;33:3734-8. [Crossref] [PubMed]
- George DA, Logoluso N, Castellini G, et al. Does cemented or cementless single-stage exchange arthroplasty of chronic periprosthetic hip infections provide similar infection rates to a two-stage? A systematic review. BMC Infect Dis 2016;16:553. [Crossref] [PubMed]
- Kildow BJ, Della-Valle CJ, Springer BD. Single vs 2-Stage Revision for the Treatment of Periprosthetic Joint Infection. J Arthroplasty 2020;35:S24-30. [Crossref] [PubMed]
- Holland G, Brown G, Goudie S, et al. Results of Using a "2-in-1" Single-Stage Revision Total Knee Arthroplasty for Infection with Associated Bone Loss: Prospective 2-Year Follow-Up. J Knee Surg 2021;34:526-32. [Crossref] [PubMed]
Cite this article as: Walmsley PJ. The ‘2-in-1’ stage: indications, technique & results. Ann Joint 2022;7:7.