
A narrative review of the biomechanical consequences of prosthesis reconstruction of the elbow
Introduction
Prosthesis reconstruction of the elbow commonly refers to one of several surgical procedures involving either hemiarthroplasty or total joint arthroplasty, each for treating a different condition. While these reconstructions can be effective treatment options to reduce pain and restore mobility to elbow joints suffering from moderate to severe arthritis or trauma in the majority of patients, poor outcomes remain a concern (1-3).
Elbow reconstruction with a prosthesis can result in different biomechanical consequences having negative impacts on joint contact mechanics, elbow kinematics, joint stability, and cartilage and/or implant articular wear, which may be a contributing factor to the relatively high complication rate and occurrence of poor patient outcomes.
This narrative review explores the biomechanical consequences associated with several elbow prosthesis reconstruction techniques, briefly summarizes their clinical impacts and patient outcomes, and discusses recent studies that investigate the biomechanical reasons for these resulting consequences. It also discusses how these issues are impacted by variables such as prosthesis design, surgical positioning, material selection, and soft tissue status, as well as hypothesizes how future research and development can attempt to improve the current state of elbow arthroplasty.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aoj-20-72).
Methods
This narrative review is based on the authors’ combined engineering expertise in these areas, consultation with leading orthopaedic surgeons, and literature review. Our literature search was performed in PubMed for supporting data, available as of February 2020, using relevant keywords; however, this was not a systematic review and does not comprehensively cover all published literature on this topic.
Discussion
Elbow hemiarthroplasty reconstruction
Elbow hemiarthroplasty is a procedure whereby only one side of the joint is replaced, often to alleviate pain and restore function following trauma to one of the articular surfaces of the elbow. Generally, hemiarthroplasty is preferred over total joint arthroplasty whenever damage is isolated to only part of a joint, as these procedures preserve more native bone, require simpler surgical procedures, and can reduce costs.
Distal humeral hemiarthroplasty (DHH)
Distal humerus fractures account for 1% of all fractures. They can occur at any age, but are more frequent in pediatric patients, patients in their second decade, elderly patients, and especially in females (4). Treatment of distal humerus fractures with open reduction and internal fixation (ORIF) and non-operative treatment may result in poor outcomes related to intolerance for joint immobilization, particularly in the context of comminuted fractures of osteoporotic bone in elderly patients, and studies have reported high incidences of complications following ORIF, such as non-union, elbow stiffness, and ulnar neuropathy (5,6). An alternative to ORIF for distal humerus fractures is DHH (Figure 1).
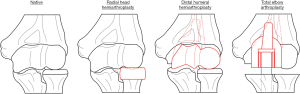
DHH replaces the native distal humeral articular surface with a metallic implant that articulates with the remaining native radial and ulnar cartilaginous surfaces. The articular component of the prosthesis is supported by a humeral stem that is implanted into the canal of the distal humerus. There are several different DHH prostheses commercially available; however, only limited clinical outcomes are available. A systematic review including 116 patients reported good to excellent results in 76.5% of non-fracture patients and only 67.4% of fracture patients, with one third of all patients experiencing a complication (1). In vitro experimental data is also lacking; particularly how the contact area and contact stress distributions in the proximal ulna cartilage layer change following DHH. How this potentially relates to cartilage erosion, is also not well understood.
Radial head hemiarthroplasty (RHH)
Radial head fractures account for about 4% of all fractures, and are a common injury of the upper limb, comprising about one third of all fractures involving the elbow (7). In the case of unreconstructible radial head fracture, radial head excision can be performed, which removes the unrepairable radial head leaving the elbow without any radiocapitellar or radioulnar articulation. Excision of the radial head can result in valgus or posterolateral instability and the potential for ulnohumeral arthrosis (7). An alternative to radial head excision in the case of an unreconstructible fracture is RHH (Figure 1).
RHH replaces only the native radial head, with a prosthesis that articulates with the intact capitellum and ulna. There are several RHH implants currently available commercially. The radiocapitellar articulation of the majority of RHH implants are axisymmetric about the radial axis, however there are several that employ a non-axisymmetric geometry. The RHH is supported by a stem inserted into the radial canal, with some implants using a loose-fit, while others accomplish a tight fit with some including bone ingrowth surfaces. Other bipolar designs permit rotation of the radial head component relative to the fixation structure using a ball in socket articulation. A report of 26 patients who received a metallic RHH showed that 50% had excellent, 17% had good and 25% had fair results at two year follow up, with the remaining 8% having poor results (2). Furthermore, 19% exhibited mild osteoarthritis on the remaining native articular cartilage.
Consequences of elbow hemiarthroplasty prosthesis reconstruction
Changes to cartilage contact mechanics
Elbow hemiarthroplasty continues to present challenges in terms of joint contact mechanics, resulting in reduced joint contact area and increased joint contact stress. This is due to the substitution of a stiff, metallic hemiarthroplasty implant in place of the native cartilage surface that has a stiffness several orders of magnitude lower (8-10). These changes in contact mechanics can result in post-operative cartilage erosion on native countersurfaces, osteoarthritis and implant failure, resulting in pain or the need for re-operation (11-16).
A review of recent advances in understanding articular cartilage tribology emphasized several factors which may contribute to cartilage erosion in hemiarthroplasty (17), which are primarily related to incompatible prosthesis stiffness and reduced geometric conformity resulting in changes in cartilage contact stresses (14,18,19). Biochemical processes may also contribute to cartilage degeneration, because abnormal stresses may promote the secretion of degenerative enzymes which cause softening and reduced elasticity of articular cartilage (20).
When comparing the intact state to a metallic DHH implant using a combined cadaveric and computational model on 8 cadaveric elbows, Lapner et al. (21) reported a significant decrease in cartilage contact area of 44% and 4% for the ulnohumeral and radiocapitellar joints, respectively. The effect of changes in DHH implant geometry and sizing on opposing cartilage contact mechanics has also been the focus of several studies. Desai et al. (22) showed in a cadaveric DHH study that optimally sized implants produced the greatest joint congruency with the ulna, with the oversized and undersized implants producing less congruency. In an effort to further improve DHH contact mechanics, anatomically derived designs reverse-engineered from the shape of the native distal humerus have been investigated. Although reverse-engineering the shape of the implant to match the native bone was experimentally shown to have a similar detrimental effect on cartilage contact area as off-the-shelf implants (23), computational modelling has shown that contact stresses are improved somewhat by these patient-specific designs; even more so when the shape accounts for the native cartilage thickness distribution (24). Subsequent computational work has shown that these designs can be further optimized by using the native bone shape as a starting point, and then uniformly increasing in size (by less than 1 mm) to compensate for the cartilage layer (25). It is worth noting, however, that reverse-engineered implants likely require accurate surgical positioning, and a study by Abhari et al. (26) found the contact mechanics were sensitive to varus-valgus positioning of the implant.
Similar results have been reported with regards to RHH implants, whereby modifications to implant geometry have been shown to alter the magnitude of changes relative to the intact state; however, all RHH implants have a significant negative impact on cartilage contact mechanics. Sahu et al. (27) tested axisymmetric and anatomically designed metallic radial head implants in 6 cadaveric elbows, and reported that while both RHH geometries significantly altered cartilage contact mechanics relative to the native state, the anatomically designed implant resulted in smaller reductions in contact area (−25% vs. −70%) and increases in mean contact stress (+29% vs. +230%) compared to the axisymmetric design. Similar results were reported in terms of peak contact stress. In an in vitro cadaveric study of 8 cadaveric elbows, Shannon et al. (28) showed that axisymmetric, quasi-anatomic, and patient specific RHH implants all significantly reduced contact area compared to the native radial head. Interestingly, they found that the general location of contact on the native radial head or RHH implant was not significantly different for any of the scenarios investigated. In a finite element contact analysis study of 15 cadaveric elbows, Langohr et al. (29) compared an axisymmetric and a non-axisymmetric RHH developed based on native radial head geometry. They found that, while the more anatomic design could significantly increase contact area and reduce maximum contact stress in certain rotational orientations compared to an axisymmetric RHH, there were also orientations that had significantly lower contact area and higher maximum contact stress. Since the radial head rotates with pronation and supination, it was deemed unlikely that the implant could be positioned such that these unfavorable orientations could be avoided completely.
The amount of allowable rotational degrees of freedom of RHH implants has also been shown to impact cartilage contact mechanics. Rotational freedom can be permitted by altering the type of implant fixation used, which varies from fixed (no rotation) to a loose over-reamed fit (increased rotational freedom). Szmit et al. (30) investigated the effect of RHH stem fit, ranging from fixed to three progressively loose fits, on cartilage contact mechanics in a finite element contact study of 10 cadaveric elbows. They reported that a moderately loose (1 to 2 mm over-reamed) fit produced the best cartilage contact mechanics for an axisymmetric RHH implant, decreasing the maximum cartilage contact stress by an average of 31%, even though mean cartilage contact area decreased as the fit was loosened. It was hypothesized that this was due to the ability of the loose fit to permit the implant to find its own optimal position based on articular contact, thus alleviating areas of impingement. Bipolar RHH designs, which incorporate a limited angle spherical joint to permit rotation of the articular component relative to the stem, also act in a similar way to allow the radial head prothesis to rotate to its optimal position, although Sahu et al. (27) also reported that bipolar RHH implants had similar joint contact area and mean contact stresses compared to standard monopolar fixed RHH implants in an in vitro cadaveric study. However, they did find that the bipolar RHH implant significantly reduced the peak cartilage contact stress by an average of 17% compared to the monopolar fixed design.
The resulting reduction in cartilage contact area and increase in contact stress following hemiarthroplasty in the elbow is clear, and while alterations in DHH and RHH implant design and geometry can affect the magnitude of these changes, even reproducing the exact cartilage geometry of the side of the joint being replaced results in significant negative consequences on contact mechanics. This is likely the result of replacing a soft cartilaginous surface with a stiff, typically metallic surface. The use of more compliant materials in elbow hemiarthroplasty has the potential to further improve the performance of these implants by further reducing the alterations in post-operative cartilage contact stress, and possibly producing similar contact mechanics as the intact state.
In a computational study of 7 cadaveric elbows, Berkmortel et al. (31) investigated cartilage contact mechanics following RHH using implants made from cobalt chrome [CoCr, Young’s modulus (E) =230,000 MPa], pyrolytic carbon (E =20,000 MPa), polyetheretherketone (PEEK, E =3,700 MPa), ultra-high molecular weight polyethylene (UHMWPE, E =690 MPa), and three grades of polycarbonate urethane (PCU) including Bionate 75D (stiff, E =290 MPa), Bionate 55F (mid, E =39 MPa), and Bionate 80A (soft, E =20 MPa). They showed that for all materials tested, the two most compliant types of PCU had significantly higher contact area and lower maximum contact stress than all other materials investigated, suggesting that elbow hemiarthroplasty could benefit from the use of an implant material having a Young’s modulus of less than 300 MPa. This was thought to be a direct result of the improved ability of the material to deform under load like that of the cartilaginous surface it is replacing, which has an aggregate stiffness of approximately 0.8 MPa (32), although the long-term wear and fatigue performance of such compliant materials still need to be assessed. Ajdari et al. (33) found similar results during in vitro testing of CoCr, ceramic, and PCU against five levels of osteoarthritic cartilage, and found that CoCr produced the most damage followed by the ceramic, while the PCU produced the least amount of damage.
Alterations to elbow kinematics
The replacement of the native distal humerus during DHH has also been shown to alter elbow kinematics due to the modification of the articular contact surfaces of the elbow. In a cadaveric study, Desai et al. (34) reported that following DHH reconstruction, both varus angulation under varus loading, and valgus angulation under valgus loading increased by up to 3° in the direction of loading compared to the native state. Interestingly, they reported that oversized implants more closely reproduced the angulation of the native elbow compared to the other sizes investigated. These changes in kinematics may further exacerbate changes in cartilage contact mechanics due to changes in load transmission at the joint. Interestingly, Sabo et al. (35) reported in a cadaveric study, that DHH of just the capitellum (and not the entire distal humerus) restored varus valgus and external rotation stability following capitellar excision. This procedure is much simpler and spares more of the native anatomy, and as such, this may have contributed to the elimination of alterations in elbow kinematics.
Total elbow arthroplasty (TEA)
When joint injury or degeneration has progressed beyond that which can be addressed using more conservative treatments, or when both sides of the joint are damaged, total joint arthroplasty is indicated.
TEA prosthesis reconstruction
TEA (Figure 1) is considered a successful procedure for restoring motion, improving function, and alleviating pain for a wide range of indications, including distal humeral nonunion, instability, established and inflammatory arthritis, and acute intra-articular comminuted fracture in selected older patients (36). It is estimated that approximately six thousand TEA procedures were performed in the US in 2015 (37). This is significantly lower than the number of lower extremity total joint arthroplasty procedures [estimated at 380 thousand primary total hips and 925 thousand primary total knees in 2015 (38)], partially due to contraindications for use in young patients because of the anticipated high rate of early failure (39).
The first TEA implants were rigid hinge designs with linked humeral and ulnar components. This design suffered from high rates of loosening, believed to be a result of high stresses at the bone-implant interface due to the constrained nature of the articulation, and has since been abandoned. Modern TEA implants feature metal on UHMWPE articulations with linked semi-constrained, unlinked, or convertible designs. Unlike the original rigid hinge designs, these devices permit some secondary motions, including internal-external (IE) and varus-valgus rotations as well as small translations. An allowable varus-valgus range of motion of 7° is common across several different designs, mimicking the coronal laxity of the intact elbow (40). Linked semi-constrained TEA implants employ a “sloppy” hinge-type linkage between the humeral and ulnar components, which allows them to be used in the presence of significant bone or ligamentous deficiencies without dislocating (41). Unlinked TEA implants rely on surrounding soft tissue integrity to stabilize the joint and prevent subluxation, and convertible designs, as the name suggest, are those which can be converted from unlinked to linked articulations intra- or post-operatively, as needed.
A study of 838 TEA recipients reported implant survival rates of 92%, 81%, 71%, and 61% at 5, 10, 15, and 20-year follow-up, respectively, with the most common reasons for revision being aseptic loosening, defective polyethylene, infection, and dislocation (3).
Alterations to elbow kinematics
The post-operative kinematics and stability of TEA depends on factors such as the congruency and shape of the articulations, whether or not a linked hinge is employed, the integrity of surrounding soft tissues (particularly the collateral ligaments), alignment and the status of the radial head.
An in vitro study by Kamineni et al. (42) examined the intrinsic constraint (varus-valgus torque versus rotation) of various designs of unlinked TEA implants in comparison with the native elbow. They reported the interesting conclusion that designs resembling the human elbow (in appearance) do not necessarily replicate normal behavior, whereas other implants which do not resemble the human elbow are able to reproduce normal behavior. A computational study by Willing et al. (43) compared the intrinsic constraint of three different linked TEA designs, and demonstrated how total laxity envelopes and torque-versus-rotation profiles can be decoupled from one-another; different profiles would influence how stable the joint feels between its laxity limits.
Valgus instability is a concern for TEA, and the likelihood of instability tends to increase with time (44). This emphasizes the importance of achieving proper valgus stability during surgery and converting to a linked TEA if there is any doubt that valgus stability is adequate. Convertible TEA designs have permitted the direct in vitro comparison of the stability of linked versus unlinked prostheses. De Vos et al. (45) confirmed that linking convertible prostheses primarily influences valgus laxity, and that linking is particularly important for valgus stability in the absence of a radial head. Collateral ligaments contribute to the stability of TEA, and their contributions towards stabilizing both linked (46) and unlinked (47) TEA has been investigated. An in vitro cadaver study by Brownhill et al. (48) investigated the effects of linking on TEA stability in collateral ligament-deficient elbows. With the collateral ligaments intact, the linked TEA had less varus-valgus laxity than in their unlinked configuration; however, these differences were not statistically significant. On the other hand, in the absence of collateral ligaments, the unlinked elbows were grossly unstable in both the varus and valgus directions. Their use of a custom instrumented stem allowed measurement of torques transmitted through the TEA articulation, which nearly doubled when a linked configuration was used, including for varus loadings which are common during many activities of daily living. This has implications regarding the potential for loosening of linked TEA, both due to the transmission of these greater forces through the stem-bone interface, as well as the increased likelihood of UHMWPE wear and related aseptic loosening.
Prosthesis alignment can also have an influence on joint biomechanics following TEA. Schuind et al. (49) measured changes in muscle moment arms after TEA with varus-valgus and IE rotational malalignments of the humeral component. IE malrotations of the humeral component were found to increase muscle moment arms and significantly alter joint kinematics, such that the linked hinge components used in their study travelled along their varus-valgus structural limits. These findings were supported by another study from the same group, which indicated that malrotations of the humeral component altered joint kinematics and forced the prosthesis to its structural limits (50). A later study by Brownhill et al. (51) found that TEA malalignment of ±6° varus-valgus and ±8° internal-external rotation resulted in changes in implant kinematics, muscle loading patterns and loads transmitted through the implant components. Thus, these studies indicate that prosthesis malalignment will alter joint biomechanics and contact mechanics of the TEA components, which could accelerate wear and loosening. Interestingly, a study by Lenoir et al. did not associate implant malalignment with loosening; however, their mean follow-up was just 23 months, and they did find that implant positioning errors seemed to affect functional outcomes, possibly due to increased stresses on soft tissues (52).
Replacement of the radial head as part of a TEA remains controversial. The radial head also plays an important role as a valgus stabilizer in the intact elbow (secondary to the medial collateral ligament) (53), and contributes to axial load bearing. Most linked and unlinked TEA involve radial head resection; although in some systems the native radial head can be preserved (54). Previous studies have measured the effects of radial head excision on unlinked TEA varus-valgus laxity, with some reporting only a small effect [e.g., Wagener et al. (55)] and others reporting a large increase in valgus laxity [e.g., Inagaki et al. (56), Ramsey et al. (57), and King et al. (47)]; these studies had similar soft-tissue management but different implant designs. Thus, the importance of the radial head relates to the intrinsic constraint of the prosthesis. For linked TER, its importance decreases further, as demonstrated by De Vos et al. (45).
Implant articular wear
Premature wear has historically been a problem for TEA prostheses, and a recent report based on data from the Norwegian Arthroplasty Register identified aseptic loosening (41.8%) and bearing damage (17.7%) as leading primary causes for TEA revision (3). Wear issues persist at the UHMWPE bushings (58), and complete deformation or wear-through can lead to unintended metal-on-metal contact. The underlying mechanisms responsible for the relatively high wear rates of TEA prostheses are not well understood. Goldberg et al. (59) presented a detailed assessment of the typical damage patterns observed on retrieved Coonrad-Morrey linked semi-constrained TEA implants and observed multiple wear modes present on all samples including damage to the humeral and ulnar polyethylene bushings showing asymmetric thinning and plastic deformation, and unintended metal-on-metal wear between bearing and nonbearing surfaces. Day et al. (60) performed detailed analyses of wear debris, which were of a size and shape shown to result in activation of cell lines involved in osteolysis (bone resorption causing loosening). Separate wear testing studies by Popoola et al. (61) and Willing (62) resulted in damage patterns on lab worn specimens under varied loading conditions that showed similarities with damage patterns of worn retrievals. More recent TEA designs feature more congruent articular surfaces and modern polyethylene blends which promise to reduce the amount damage due to wear.
Summary
Post-operative changes in cartilage contact area and stress following DHH and RHH are affected by implant articular geometry and material. Clearly the replacement of one side of a cartilage-on-cartilage joint with a stiff material results in negative consequences in terms of reduced articular contact area and increased contact stress. The optimization of articular geometry through further computational and in vitro investigation combined with the use of softer, more complaint materials may improve elbow hemiarthroplasty outcomes, although the long-term wear and fatigue performance of such compliant materials needs to be further investigated in the application of hemiarthroplasty. In addition to alterations in cartilage contact mechanics, hemiarthroplasty has also been shown to have the potential to alter elbow kinematics due to the replacement of native geometry with an implant, which may further exacerbate cartilage degeneration due to changes in load transfer at the joint.
TEA is capable of restoring native elbow kinematics; however, the functional stability of the joint is dependent on surrounding soft tissues, implant shape and the degree of constraint selected for use. In the case of healthy soft tissue in the elbow, an unlinked design can be sufficient, however if the collateral ligaments are deficient, a linked design can help to mitigate stability issues. Similarly, radial head condition can affect stability, particularly for unlinked implants which rely on this structure to provide stability, particularly in the case when collateral ligaments are deficient. This is less important in the use of linked designs, as they are capable of supporting varus-valgus moments. TEA wear continues to be an issue, as many previous designs which are still in patients today exhibited high articular contact stresses resulting in wear and deformation of the polymer articulation. This effect may have been mitigated with the introduction of more congruent designs and the inclusion of advanced materials, but the long-term performance of these changes is yet to be determined.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Spencer P. Lake) for the series “Emerging Trends in Elbow Injury, Pathology and Treatment” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aoj-20-72
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-72). The series “Emerging Trends in Elbow Injury, Pathology and Treatment” was commissioned by the editorial office without any funding or sponsorship. RTW reports grants from LIMA Corp., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dunn J, Kusnezov N, Pirela-Cruz M. Distal humeral hemiarthroplasty: indications, results, and complications. A systematic review. Hand 2014;9:406-12. [Crossref] [PubMed]
- Grewal R, MacDermid JC, Faber KJ, et al. Comminuted radial head fractures treated with a modular metallic radial head arthroplasty. Study of outcomes. J Bone Joint Surg Am 2006;88:2192-200. [Crossref] [PubMed]
- Krukhaug Y, Hallan G, Dybvik E, et al. A survivorship study of 838 total elbow replacements: a report from the Norwegian Arthroplasty Register 1994-2016. J Shoulder Elbow Surg 2018;27:260-9. [Crossref] [PubMed]
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury 2006;37:691-7. [Crossref] [PubMed]
- McKee MD, Veillette CJ, Hall JA, et al. A multicenter, prospective, randomized, controlled trial of open reduction--internal fixation versus total elbow arthroplasty for displaced intra-articular distal humeral fractures in elderly patients. J Shoulder Elbow Surg 2009;18:3-12. [Crossref] [PubMed]
- Korner J, Lill H, Müller LP, et al. Distal humerus fractures in elderly patients: results after open reduction and internal fixation. Osteoporos Int 2005;16 Suppl 2:S73-9. [Crossref] [PubMed]
- Duckworth AD, McQueen MM, Ring D. Fractures of the radial head. Bone Joint J 2013;95-B:151-9. [Crossref] [PubMed]
- Sabo MT, Shannon H, Ng J, et al. The impact of capitellar arthroplasty on elbow contact mechanics: Implications for implant design. Clin Biomech (Bristol, Avon) 2011;26:458-63. [Crossref] [PubMed]
- Shannon HL. The Contact Mechanics and Kinematics of Radial Head Implants. University of Western Ontario, 2012.
- Liew VS, Cooper IC, Ferreira LM, et al. The effect of metallic radial head arthroplasty on radiocapitellar joint contact area. Clin Biomech (Bristol, Avon) 2003;18:115-8. [Crossref] [PubMed]
- Popovic N, Lemaire R, Georis P, et al. Midterm results with a bipolar radial head prosthesis: radiographic evidence of loosening at the bone-cement interface. J Bone Joint Surg Am 2007;89:2469-76. [PubMed]
- Burkhart KJ, Mattyasovszky SG, Runkel M, et al. Mid- to long-term results after bipolar radial head arthroplasty. J Shoulder Elbow Surg 2010;19:965-72. [Crossref] [PubMed]
- Harrington IJ, Sekyi-Otu A, Barrington TW, et al. The functional outcome with metallic radial head implants in the treatment of unstable elbow fractures: a long-term review. J Trauma 2001;50:46-52. [Crossref] [PubMed]
- van der Meulen MC, Beaupré GS, Smith RL, et al. Factors influencing changes in articular cartilage following hemiarthroplasty in sheep. J Orthop Res 2002;20:669-75. [Crossref] [PubMed]
- Lombardi AV Jr, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty. Orthopedics 2008;31:927-30. [Crossref] [PubMed]
- Smith GC, Hughes JS. Unreconstructable acute distal humeral fractures and their sequelae treated with distal humeral hemiarthroplasty: a two-year to eleven-year follow-up. J Shoulder Elbow Surg 2013;22:1710-23. [Crossref] [PubMed]
- Katta J, Jin Z, Ingham E, et al. Biotribology of articular cartilage--a review of the recent advances. Med Eng Phys 2008;30:1349-63. [Crossref] [PubMed]
- LaBerge M, Bobyn JD, Drouin G, et al. Evaluation of metallic personalized hemiarthroplasty: a canine patellofemoral model. J Biomed Mater Res 1992;26:239-54. [Crossref] [PubMed]
- McCann L, Ingham E, Jin Z, et al. An investigation of the effect of conformity of knee hemiarthroplasty designs on contact stress, friction and degeneration of articular cartilage: a tribological study. J Biomech 2009;42:1326-31. [Crossref] [PubMed]
- Moon KH, Kang JS, Lee TJ, et al. Degeneration of acetabular articular cartilage to bipolar hemiarthroplasty. Yonsei Med J 2008;49:719-24. [Crossref] [PubMed]
- Lapner M, Willing R, Johnson JA, et al. The effect of distal humeral hemiarthroplasty on articular contact of the elbow. Clin Biomech (Bristol, Avon) 2014;29:537-44. [Crossref] [PubMed]
- Desai SJ, Lalone E, Athwal GS, et al. Hemiarthroplasty of the elbow: the effect of implant size on joint congruency. J Shoulder Elbow Surg 2016;25:297-303. [Crossref] [PubMed]
- Willing R, Lapner M, King GJWW, et al. In vitro assessment of the contact mechanics of reverse-engineered distal humeral hemiarthroplasty prostheses. Clin Biomech (Bristol, Avon) 2014;29:990-6. [Crossref] [PubMed]
- Willing R, King GJW, Johnson JA. Contact mechanics of reverse engineered distal humeral hemiarthroplasty implants. J Biomech 2015;48:4037-42. [Crossref] [PubMed]
- Willing R. Design optimisation improves the performance of custom distal humeral hemiarthroplasty implants. Comput Methods Biomech Biomed Eng Imaging Vis 2019;7:108-15. [Crossref]
- Abhari RE, Willing R, King GJW, et al. An In Vitro Study of the Role of Implant Positioning on Ulnohumeral Articular Contact in Distal Humeral Hemiarthroplasty. J Hand Surg Am 2017;42:602-9. [Crossref] [PubMed]
- Sahu D, Holmes DM, Fitzsimmons JS, et al. Influence of radial head prosthetic design on radiocapitellar joint contact mechanics. J Shoulder Elbow Surg 2014;23:456-62. [Crossref] [PubMed]
- Shannon HL, Deluce SR, Lalone EA, et al. Effect of radial head implant shape on joint contact area and location during static loading. J Hand Surg Am 2015;40:716-22. [Crossref] [PubMed]
- Langohr GDG, Willing RT, Medley JB, et al. Contact analysis of the native radiocapitellar joint compared with axisymmetric and nonaxisymmetric radial head hemiarthroplasty. J Shoulder Elbow Surg 2015;24:787-95. [Crossref] [PubMed]
- Szmit J, King GJW, Johnson JA, et al. The effect of stem fit on the radiocapitellar contact mechanics of a metallic axisymmetric radial head hemiarthroplasty: is loose fit better than rigidly fixed? J Shoulder Elbow Surg 2019;28:2394-9. [Crossref] [PubMed]
- Berkmortel C, Langohr GDG, King G, et al. Hemiarthroplasty implants should have very low stiffness to optimize cartilage contact stress. J Orthop Res 2020;38:1719-26. [Crossref] [PubMed]
- Langohr GDG, Willing R, Medley JB, et al. The Effect of Radial Head Hemiarthroplasty Geometry on Radiocapitellar Joint Contact Mechanics. J Shoulder Elb Surg 2015;24:e118. [Crossref]
- Ajdari N, Tempelaere C, Masouleh MI, et al. Hemiarthroplasties: the choice of prosthetic material causes different levels of damage in the articular cartilage. J Shoulder Elbow Surg 2020;29:1019-29. [Crossref] [PubMed]
- Desai SJ. Distal Humerus Hemiarthroplasty: Joint Kinematics, Stability, Congruency and Implant Design. Electronic Thesis and Dissertation Repository, 2015:2671.
- Sabo MT, Shannon HL, Deluce S, et al. Capitellar excision and hemiarthroplasty affects elbow kinematics and stability. J Shoulder Elbow Surg 2012;21:1024-1031.e4. [Crossref] [PubMed]
- Lee BP, Adams RA, Morrey BF. Polyethylene wear after total elbow arthroplasty. J Bone Joint Surg Am 2005;87:1080-7. [Crossref] [PubMed]
- Day JS, Lau E, Ong KL, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010;19:1115-20. [Crossref] [PubMed]
- Kurtz SM, Ong KL, Lau E, et al. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624-30. [Crossref] [PubMed]
- Celli A, Morrey BF. Total elbow arthroplasty in patients forty years of age or less. J Bone Joint Surg Am 2009;91:1414-8. [Crossref] [PubMed]
- Ramsey ML, Adams RA, Morrey BF. Instability of the elbow treated with semiconstrained total elbow arthroplasty. J Bone Joint Surg Am 1999;81:38-47. [Crossref] [PubMed]
- Cil A, An KN, O'Driscoll SW. Custom triflange outrigger ulnar component in revision total elbow arthroplasty. J Shoulder Elbow Surg 2011;20:192-8. [Crossref] [PubMed]
- Kamineni S, O'Driscoll SW, Urban M, et al. Intrinsic constraint of unlinked total elbow replacements--the ulnotrochlear joint. J Bone Joint Surg Am 2005;87:2019-27. [Crossref] [PubMed]
- Willing R, King GJ, Johnson JA. The effect of implant design of linked total elbow arthroplasty on stability and stress: a finite element analysis. Comput Methods Biomech Biomed Engin 2014;17:1165-72. [Crossref] [PubMed]
- Voloshin I, Schippert DW, Kakar S, et al. Complications of total elbow replacement: A systematic review. J Shoulder Elbow Surg 2011;20:158-68. [Crossref] [PubMed]
- De Vos MJ, Wagener ML, Hendriks JCM, et al. Linking of total elbow prosthesis during surgery; A biomechanical analysis. J Shoulder Elbow Surg 2013;22:1236-41. [Crossref] [PubMed]
- Herren DB, O’Driscoll SW, An KN. Role of collateral ligaments in the GSB-linked total elbow prosthesis. J Shoulder Elbow Surg 2001;10:260-4. [Crossref] [PubMed]
- King GJ, Itoi E, Niebur GL, et al. Motion and laxity of the capitellocondylar total elbow prosthesis. J Bone Joint Surg Am 1994;76:1000-8. [Crossref] [PubMed]
- Brownhill JR, Pollock JW, Ferreira LM, et al. The effect of implant linking and ligament integrity on humeral loading of a convertible total elbow arthroplasty. Shoulder Elbow 2019;11:45-52. [Crossref] [PubMed]
- Schuind F, O’Driscoll SW, Korinek S, et al. Changes of elbow muscle moment arms after total elbow arthroplasty. J Shoulder Elbow Surg 1994;3:191-9. [Crossref] [PubMed]
- Schuind F, O’Driscoll S, Korinek S, et al. Loose-hinge total elbow arthroplasty: An experimental study of the effects of implant alignment on three-dimensional elbow kinematics. J Arthroplasty 1995;10:670-8. [Crossref] [PubMed]
- Brownhill JR, Pollock JW, Ferreira LM, et al. The effect of implant malalignment on joint loading in total elbow arthroplasty: An in vitro study. J Shoulder Elbow Surg 2012;21:1032-8. [Crossref] [PubMed]
- Lenoir H, Micallef JP, Djerbi I, et al. Total elbow arthroplasty: Influence of implant positioning on functional outcomes. Orthop Traumatol Surg Res 2015;101:721-7. [Crossref] [PubMed]
- Morrey BF, Tanaka S, An KN. Valgus stability of the elbow. A definition of primary and secondary constraints. Clin Orthop Relat Res 1991.187-95. [PubMed]
- Lingenfelter EJ, Adams RA, Morrey B. Management of the radial head and linked total elbow arthroplasty. J Shoulder Elbow Surg 2011;20:625-30. [Crossref] [PubMed]
- Wagener ML, De Vos MJ, Hendriks JCM, et al. Stability of the unlinked Latitude total elbow prosthesis: A biomechanical in vitro analysis. Clin Biomech (Bristol, Avon) 2013;28:502-8. [Crossref] [PubMed]
- Inagaki K, O'Driscoll SW, Neale PG, et al. Importance of a radial head component in Sorbie unlinked total elbow arthroplasty. Clin Orthop Relat Res 2002.123-31. [Crossref] [PubMed]
- Ramsey M, Neale PG, Morrey BF, et al. Kinematics and functional characteristics of the Pritchard ERS unlinked total elbow arthroplasty. J Shoulder Elbow Surg 2003;12:385-90. [Crossref] [PubMed]
- Throckmorton T, Zarkadas P, Sanchez-Sotelo J, et al. Failure patterns after linked semiconstrained total elbow arthroplasty for posttraumatic arthritis. J Bone Joint Surg Am 2010;92:1432-41. [Crossref] [PubMed]
- Goldberg SH, Urban RM, Jacobs JJ, et al. Modes of wear after semiconstrained total elbow arthroplasty. J Bone Joint Surg Am 2008;90:609-19. [Crossref] [PubMed]
- Day JS, Baxter RM, Ramsey ML, et al. Characterization of wear debris in total elbow arthroplasty. J Shoulder Elbow Surg 2013;22:924-31. [Crossref] [PubMed]
- Popoola OO, Kincaid BL, Mimnaugh K, et al. In vitro wear of ultrahigh-molecular-weight polyethylene and vitamin E blended highly cross-linked polyethylene in linked, semiconstrained total elbow replacement prostheses. J Shoulder Elbow Surg 2017;26:846-54. [Crossref] [PubMed]
- Willing R. Comparing damage on retrieved total elbow replacement bushings with lab worn specimens subjected to varied loading conditions. J Orthop Res 2018;36:1998-2006. [Crossref] [PubMed]
Cite this article as: Langohr GDG, Willing RT. A narrative review of the biomechanical consequences of prosthesis reconstruction of the elbow. Ann Joint 2021;6:6.


