Classification and management options for prosthetic joint infection
Classification of prosthetic joint infections
Classification schemes for periprosthetic joint infections (PJIs) have been shown to be beneficial in terms of predicting the most appropriate treatment strategy.
The most widely accepted classification of periprosthetic infections of total joint replacements has been proposed by Coventry (1) and divides the occurrence of the infection into three stages: stage I (acute, within the first three months); stage II (more than 3 months after surgery); stage III (2 years after infection) (Table 1). Perhaps the most frequently cited classification of PJI is the one formulated by Tsukayama et al. (2) which proposed a system which categorized infections into four groups (Table 2):
Table 1
| Type I | Type II | Type III | |
|---|---|---|---|
| Presentation | Acute postoperative infection | Late chronic infection | Late hematogenous infection |
| Definition | Acute infection within the first 30 days after surgery | Chronic indolent infection presenting more than 30 days after surgery | Presenting beyond 2 years |
PJI, periprosthetic joint infection.
Table 2
| Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|
| Timing | Positive intraoperative culture | Early postoperative infection | Acute hematogenous infection | Late (chronic) infection |
| Clinical presentation | More than 2 positive intraoperative cultures | Infection occurring within first month | Hematogenous seeding of site of previously well-functioning prosthesis | Chronic indolent clinical course; infection present for more than 30 days |
PJI, periprosthetic joint infection.
- Positive intra-operative cultures;
- Early postoperative infection occurring before 4 weeks;
- Late chronic infection (>4 weeks), and;
- Acute hematogenous infection.
A similar system was proposed by Toms et al. (3), who otherwise consider early postoperative infections those being detected <6 weeks. Similarly, Cui et al. classified infection into four types according to onset of symptoms and positivity to intra-operative cultures (4).
Zimmerli et al. (5), distinguish between early PJIs (within 3 months postoperatively), delayed (3 to 24 months) or late (more than 24 months). A period of 3 months after surgery as the cut-off between acute or not has been mentioned also by Parvizi et al. (6). Recent recommendations reported by Osmon et al. (7) have achieved widespread acceptance internationally.
The timing of intervention is important. A short duration of symptoms is commonly considered the best prognostic factor in terms of eradication of infection (8,9). However, clarifications are needed in borderline cases, as the cut-off of an acute PJI ranges between 0–4 weeks (2) and 0–3 months (5,6). Based on available literature, robust scientific evidence correlating the duration of symptoms with the clinical outcome is lacking.
For these reasons a comprehensive seven point PJI classification has been proposed by Romanò et al. (10). This classification system focuses on several issues including the host status, responsible microorganisms, bone and soft tissue defects, aetiopathogenesis, and anatomical and pathological features, from acute, with rapid-onset pain, swelling and wound purulence with or without systemic features of infection, to chronic, with serious discomfort, decreased movement and presence of sinus tract.
This system seems intuitive as, given that multiple factors influence PJI, it is illogical that timing alone influences management and outcomes.
More recently, in order to address ambiguities arising in current guidelines ant to encompass the cases which do not conform to the available classification systems, a different perspective has been introduced (11) (Table 3).
Table 3
| Type I | Type II | Type III | Type IV | |
|---|---|---|---|---|
| Presentation | Acute postoperative infection | Acute postoperative infection | Chronic infection | Chronic infection |
| Location | Joint space | Bone/implant interface | Joint space | Bone/implant interface |
PJI, periprosthetic joint infection.
This new classification proposal focuses on the identification of different patterns of infection based on the topography of the infectious process: this theory relies on the identification of the exact location of the bacterial colonization and provides guidance for the treating surgeons, allowing them to decide between a conservative or a more radical intervention irrespectively of the timing (11).
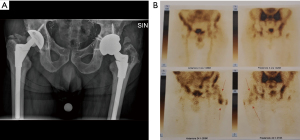
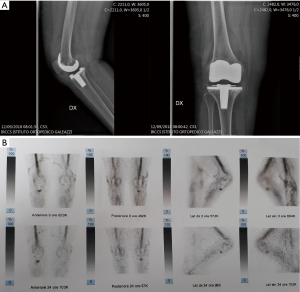
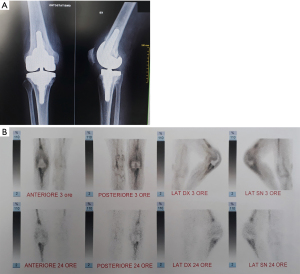
In order to accurately localize the focus of infection contemporary imaging modalities such as nuclear scanning may be used; which may allow to improve localization and to better understand PJI patterns compared to conventional radiographs. Radiolabeled white blood cells (WBC) imaging, possesses the accuracy to distinguish between septic and aseptic loosening (12-14). Single-photon emission computed tomography (SPECT) is currently overtaking planar scintigraphy with a more detailed 3D localization (15), and the recent introduction of integrated SPECT/CT has allowed a more precise anatomic localization (16).
With this idea in mind, by using radiolabeled WBC by SPECT/CT to accurately localize the focus of infection, three different patterns can be identified:
- Infection involving the joint space (Figure 1);
- Infection involving the bone-implant interface (Figure 2);
- Infection involving both compartments (Figure 3).
This new approach, by taking advantage of recent nuclear imaging modalities, could improve current management of PJIs allowing useful selection criteria enhancing therapeutic strategies (Figure 4).
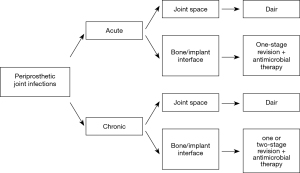
Management options
Several factors related to the host, infecting species and the surgeon influence the choice of treatment. Irrespective of the classification used the management should focus on eradicating the infection and restoring the pain free function of the affected limb. At present, treatment strategies for PJIs are based on the progression of the infectious process and clinical involvement. Long term antibiotic suppression therapy is an option if surgical treatment it precluded (17).
Current surgical management relies on debridement, antibiotics and implant retention (DAIR), which is traditionally performed at early stages following the onset of symptoms, 1- or 2-stage revision arthroplasty which is usually limited to chronic stages. In addition, in severe cases, definitive articulating antibiotic spacer (shoulder), excision arthroplasty (hip), arthrodesis or amputation (knee) can be performed.
Typically, DAIR is considered to be the treatment of choice in patients with a short duration of symptoms, which allows implant preservation, good functional outcomes and shorter hospital stays (18-26).
A 2-stage exchange strategy is commonly considered to be the ‘gold standard’ for the management of PJI, since it is advocated to provide effective infection eradication, although it is a complex surgical procedure usually which can itself result in bone and soft tissue damage (27).
The one-stage approach possesses the advantages of avoiding multiple major invasive surgical procedures and prolonged hospitalization however it needs strict criteria to be applied as it has to be performed in healthy patients Type A hosts (1) with healthy soft tissues and with minimal or moderate bone loss and in whom the infecting organism and the antibiotic sensitivities are known.
Several authors have reported similar rates of infection recurrence following one and two-stage revisions (28-32), and the use of one-stage revision surgery is gaining popularity.
Recently, satisfying results following partial implant retention during revision total arthroplasty for septic failures have been reported (33-36).
In a study by Ekpo et al. (33), a success rate of 89.4% (17 out of 19) has been reported at a minimum of 2 years follow-up after partial revision arthroplasty (range, 2–11 years). Similarly, a low reinfection rate (6.6%) has been reported by Morley et al. (34) 6.8 years following partial hip revision surgery. El-Husseiny et al. (36) reviewed 18 patients with infected THAs treated with selective implant retention at a minimum of 5 years follow-up (range, 5–9.9 years). Three patients (16.6%) had recurrent infection at the site of the prosthesis. Postoperative average Harris hip score was 78 (range, 46–89). These positive outcomes may demonstrate that bacteria have not invaded all implant components, and that the identification of the exact location of the infection may allow selective implant retention (11).
The use of a permanent cement spacer and resection arthroplasty may be considered viable treatment options in elderly, low-demand patient with severe medical comorbidities, as well as patients with limited bone stock, poor soft tissue coverage, or infections due to highly resistant organisms in whom surgery is deemed too high risk (37-41).
Similarly arthrodesis represents a salvage option for the septic prosthetic knee joint infection once multiple revision procedures have been exhausted providing acceptable functionality and satisfactory quality of life when bone stock is insufficient (40,41).
In case no measures to salvage a functional TKA can be pursued, knee arthrodesis or above-knee amputation should be considered as salvage procedures to eradicate the infectious process, and sometimes saves the patients’ life. Knee arthrodesis may allow limb preservation and residual joint functionality in absence of sufficient bone stock (42). Amputation should be the last option considered in presence of serious and permanent tissue damage, although may be appropriate in selected cases (43,44).
Conclusions
The classification of PJI can be used to guide clinicians with therapeutic decision making. There are however several classification systems which vary in their definition of what constitutes an acute infection as well as what prognostic factors are important in patients with PJI. Multicentre prospective randomized controlled trials are required to help to define these important issues.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nemandra A. Sandiford, Massimo Francescini and Daniel Kendoff) for the series “Prosthetic Joint Infection” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-86). The series “Prosthetic Joint Infection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coventry MB. Treatment of infections occurring in total hip surgery. Orthop Clin North Am 1975;6:991-1003. [Crossref] [PubMed]
- Tsukayama DT, Estrada R, Gustilo R. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996;78:512-23. [Crossref] [PubMed]
- Toms AD, Davidson D, Masri BA, et al. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br 2006;88:149-55. [Crossref] [PubMed]
- Cui Q, Mihalko WM, Shields JS, et al. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007;89:871-82. [Crossref] [PubMed]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645-54. [Crossref] [PubMed]
- Parvizi J, Adeli B, Zmistowski B, et al. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am 2012;94:e104. [Crossref] [PubMed]
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1-10. [Crossref] [PubMed]
- Romanò CL, Manzi G, Logoluso N, et al. Value of debridement and irrigation for the treatment of peri-prosthetic infections. A systematic review. Hip Int 2012;S19-24. [Crossref] [PubMed]
- Azzam KA, Seeley M, Ghanem E, et al. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty 2010;25:1022-7. [Crossref] [PubMed]
- Romanò CL, Romano D, Logoluso N. Bone and joint infection on adults: a comprehensive classification proposal. Eur Orthop Traumatol 2011;1:207-17. [Crossref] [PubMed]
- Pellegrini A, Legnani C, Meani E. A new perspective on current prosthetic joint infection classifications: introducing topography as a key factor affecting treatment strategy. Arch Orthop Trauma Surg 2019;139:317-22. [Crossref] [PubMed]
- Palestro CJ. Radionuclide Imaging of Musculoskeletal Infection: A Review. J Nucl Med 2016;57:1406-12. [Crossref] [PubMed]
- Palestro CJ. Nuclear medicine and the failed joint replacement: past, present, and future. World J Radiol 2014;6:446-8. [Crossref] [PubMed]
- Signore A, Sconfienza LM, Borens O, et al. Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging 2019;46:971-88. [Crossref] [PubMed]
- van der Bruggen W, Bleeker-Rovers CP, Boerman OC, et al. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: a systematic review. Semin Nucl Med 2010;40:3-15. [Crossref] [PubMed]
- Kim HO, Na SJ, Oh SJ, et al. Usefulness of adding SPECT/CT to 99mTchexamethylpropylene amine oxime (HMPAO)-labeled leukocyte imaging for diagnosing prosthetic joint infections. J Comput Assist Tomogr 2014;38:313-9. [Crossref] [PubMed]
- Prendki V, Sergent P, Barrelet A, et al. Efficacy of indefinite chronic oral antimicrobial suppression for prosthetic joint infection in the elderly: a comparative study. Int J Infect Dis. 2017;60:57-60. [Crossref] [PubMed]
- Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res 2002;125-31. [Crossref] [PubMed]
- Deirmengian C, Greenbaum J, Lotke PA, et al. Limited success with open debridement and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J Arthroplasty 2003;18:22-6. [Crossref] [PubMed]
- Flierl MA, Culp BM, Okroj KT, et al. Poor Outcomes of Irrigation and Debridement in Acute Periprosthetic Joint Infection With Antibiotic-Impregnated Calcium Sulfate Beads. J Arthroplasty 2017;32:2505-7. [Crossref] [PubMed]
- Romanò C, Logoluso N, Drago L, et al. Role for irrigation and debridement in periprosthetic infections. J Knee Surg 2014;27:267-72. [Crossref] [PubMed]
- Lizaur-Utrilla A, Gonzalez-Parreno S, Gil-Guillen V, et al. Debridement with prosthesis retention and anthibiotherapy vs two-stage revision for periprosthetic knee infection within 3 months after arthroplasty: a case-control study. Cin Microbiol Infect 2015;21:851.e11-7. [Crossref]
- Urish KL, Bullock AG, Kreger AM, et al. A Multicenter Study of Irrigation and Debridement in Total Knee Arthroplasty Periprosthetic Joint Infection: Treatment Failure Is High. J Arthroplasty 2018;33:1154-9. [Crossref] [PubMed]
- Gardner J, Gioe TJ, Tatman P. Can this prosthesis be saved? Implant salvage attempts in infected primary TKA. Clin Orthop Relat Res 2011;469:970-6. [Crossref] [PubMed]
- Fehring TK, Odum SM, Berend KR, et al. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin Orthop Relat Res 2013;471:250-7. [Crossref] [PubMed]
- Mont MA, Waldman B, Banerjee C, et al. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty 1997;12:426-33. [Crossref] [PubMed]
- Berend KR, Lombardi AV Jr, Morris MJ, et al. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res 2013;471:510-8. [Crossref] [PubMed]
- Buechel FF, Femino FP, D’Alessio J. Primary exchange revision arthroplasty for infected total knee replacement: a longterm study. Am J Orthop (Belle Mead NJ) 2004;33:190-8. [PubMed]
- Jenny JY, Barbe B, Gaudias J, et al. High infection control rate and function after routine one-stage exchange for chronically infected TKA. Clin Orthop Relat Res 2013;471:238-43. [Crossref] [PubMed]
- Wongworawat MD. Clinical faceoff: One- versus two-stage exchange arthroplasty for prosthetic joint infections. Clin Orthop Relat Res 2013;471:1750-3. [Crossref] [PubMed]
- Nagra NS, Hamilton TW, Ganatra S, et al. One‑stage versus two‑stage exchange arthroplasty for infected total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2016;24:3106-14. [Crossref] [PubMed]
- Pellegrini A, Legnani C, Macchi V, et al. Two-stage revision shoulder prosthesis vs. permanent articulating antibiotic spacer in the treatment of periprosthetic shoulder infections. Orthop Traumatol Surg Res 2019;105:237-40. [Crossref] [PubMed]
- Ekpo TE, Berend KR, Morris MJ, et al. Partial two-stage exchange for infected total hip arthroplasty: a preliminary report. Clin Orthop Relat Res 2014;472:437-48. [Crossref] [PubMed]
- Morley JR, Blake SM, Hubble MJ, et al. Preservation of the original femoral cement mantle during the management of infected cemented total hip replacement by two-stage revision. J Bone Joint Surg Br 2012;94:322-7. [Crossref] [PubMed]
- Lee YK, Lee KH, Nho JH, et al. Retaining well-fixed cementless stem in the treatment of infected hip arthroplasty. Acta Orthop 2013;84:260-4. [Crossref] [PubMed]
- El-Husseiny M, Haddad FS. The Role of Highly Selective Implant Retention in the Infected Hip Arthroplasty. Clin Orthop Relat Res 2016;474:2157-63. [Crossref] [PubMed]
- Pellegrini A, Legnani C, Macchi V, et al. Management of periprosthetic shoulder infections with the use of a permanent articulating antibiotic spacer. Arch Orthop Trauma Surg 2018;138:605-9. [Crossref] [PubMed]
- Fink B, Sevelda F. Periprosthetic Joint Infection of Shoulder Arthroplasties: Diagnostic and Treatment Options. Biomed Res Int 2017;2017:4582756. [Crossref] [PubMed]
- Fleck EE, Spangehl MJ, Rapuri VR, et al. An articulating antibiotic spacer controls infection and improves pain and function in a degenerative septic hip. Clin Orthop Relat Res 2011;469:3055-64. [Crossref] [PubMed]
- Abblitt WP, Ascione T, Bini S, et al. Hip and Knee Section, Outcomes: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019;34:S487-95. [Crossref] [PubMed]
- Balato G, Rizzo M, Ascione T, et al. Re-infection rates and clinical outcomes following arthrodesis with intramedullary nail and external fixator for infected knee prosthesis: a systematic review and meta-analysis. BMC Musculoskelet Disord 2018;19:361. [Crossref] [PubMed]
- Wu CH, Gray CF, Lee GC. Arthrodesis should be strongly considered after failed two-stage reimplantation TKA. Clin Orthop Relat Res 2014;472:3295-304. [Crossref] [PubMed]
- Gehrke T, Alijanipour P, Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J 2015;97-B:20-9. [Crossref] [PubMed]
- Hawi N, Kendoff D, Citak M, et al. Septic single-stage knee arthrodesis after failed total knee arthroplasty using a cemented coupled nail. Bone Joint J 2015;97-B:649-53. [Crossref] [PubMed]
Cite this article as: Pellegrini A, Suardi V, Legnani C. Classification and management options for prosthetic joint infection. Ann Joint 2022;7:3.