
Two stage revision: indications, techniques and results
Introduction
Periprosthetic joint infection (PJI) is one of the most challenging complications a surgeon has to face after prosthetic replacement of a joint and one of the most devastating complications for the patient. Every year the number of joint replacements becomes greater. It has been predicted that there will be an increase of 85% in total knee replacements (1.26 million) and a 71% increase in total hip replacements [635,000] by 2030 (1). This expectation is related to the average increase in life expectancy and in expansions of indications in both in younger and in older patients. According to an analysis by the World Health Organization between 2000 and 2016, there was an average increase in life expectancy of 5.5 years (2). The risk of developing a PJI following primary total knee and hip replacement varies between 0.5% and 2% however the risk following revision total joint arthroplasty has been reported to be as high as 20% (3-5). The risk of reinfection following surgical intervention for PJI 7.6% and 8.8% for single-stage and two-stage reviews, respectively (6). For these reasons the subject of PJI is likely to become a bigger issue over time. The optimal surgical approach for the management of PJI is undecided however the two-stage approach has been considered the gold standard.
The aim of this review is to analyze the current literature on two-stage revision in hip and knee periprosthetic infections with the aim of providing concise data to clinicians involved in the management PJI.
Diagnosis and classification of PJI
Prosthetic joint infection should always be considered as a potential cause of a failing prosthesis (3). The American Academy of Orthopaedic Surgeons (AAOS) introduced theoretical and practical guidelines for the diagnosis of PJI in 2010 (7). During the 2018 International Consensus Meeting (ICM) in Philadelphia a numerical diagnostic tool was proposed consisting of two major criteria and eight minor criteria (Figure 1). The presence of two positive cultures with the use of standard sampling methods or the evidence of a fistula in communication with the joint (or even the exposure of the prosthesis) have been defined as major criteria for the diagnosis of certainty of periprosthetic infection (8). In 2019 Shohat et al. proposed a diagnostic algorithm based on the principles dictated during the 2018 ICM (9,10). Toms and colleagues described 4 modes of presentation and proposed a classification based on this (11).
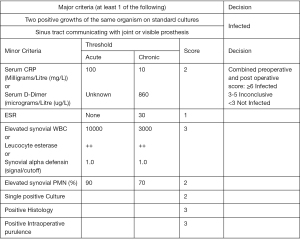
- Stage 1: acute infections that occurs within 6 weeks;
- Stage 2: a delayed presentation with a chronic indolent infection;
- Stage 3: those that occur suddenly in a well-functioning implant with an acute presentation secondary to haematogenous spread;
- Stage 4: when a positive culture is found at the time of surgery without a previous evidence of infection.
Indications for surgical management
Several authors have attempted to provide robust indications for specific surgical treatment options. Wouthuyzen-Bakker et al. attempted to provide a decision algorithm based on the CRIME80-score (i.e., CRP >150 mg/L, Chronic obstructive pulmonary disease, rheumatoid arthritis, fracture as Indication for the prosthesis, male gender, not exchanging the mobile components during debridement and an age above 80 years). By calculating the probability of the failure of the debridement and implant retention (DAIR), they concluded that a possibility of failure of the DAIR between 50–65% can indicate the need for exchange arthroplasty. If the percentage of failure exceeds 65% then it is mandatory to remove the implant and proceed to a revision (12). A recent study by Kunutsor et al. compared data from patients undergoing one- vs. two-stage hip replacement revision. They concluded that there is no significant difference in outcomes, but underlines that there are strong limitations regarding the analysis of the prognostic factors for the success of single versus 2 stage revision (13).
Contraindications to single stage revision include significant soft tissue injury with the inability to provide soft tissue cover, the presence of unknown or multi resistant organisms, lack of access to appropriate antibiotics or lack of appropriate expertise (14). Two stage revision surgery is indicated in these situations.
Surgical technique
Insall et al. described the surgical two-stage prosthetic revision technique in 1983 (15) and subsequently in 1995 Garvin and Hanssen (16) conducted a literature review demonstrating the success of the surgical technique.
Two stage revision is a technique in which the in situ prosthesis is removed, a thorough debridement of bone and soft tissue is performed, an interval spacer is inserted, antibiotics administered. The 2nd stage of definitive reconstruction is performed once the infection is deemed to have resolved. The interval between stages can range from 6 weeks to several months. Careful and judicious debridement of all necrotic and infected tissue is required at both stages.
First stage
The first stage consists of the removal of all indwelling metalwork and cement (if present) and an accurate and aggressive debridement of the entire effective joint space. if possible antibiotics are withheld until tissue samples have been harvested. These are sent for histological as well as microbial analysis. It’s recommended to remove the previous scar and if present the fistula. The prosthetic components may be sent for sonification. This requires special packaging and should be planned prior to surgery.
It’s very important to remove all soft tissues which is macroscopically involved in the infection process and all the cement, even if well fixed to the underlying bone (10). Cement can be removed with osteotomes, or specialized chisels, drills and taps or with the use of other techniques which utilize ultrasound based extraction instrumentation (17). The surgeon has to be cautious during this stage as there is a risk of iatrogenic injury to the bone. Foci of heterotopic ossification should be removed if possible (10). Copious lavage is required with a minimum of 6 litres of fluid using a high-pressure pulsatile lavage system. The authors’ preferred fluid is normal saline. This has an important mechanical action which physically removes sequestra, necrotic tissue and micro-organisms as a well as dilution. Several authors have investigated addition of antibiotics to this solution however no clinical benefit has been demonstrated over plain lavage solution.
Once the explantation and debridement stage is completed, new sterile drapes are applied over the existing ones and the spacer is inserted. Spacers may be static or dynamic, preformed or handmade, hemi arthroplasty of replace both sides of the joint. Appropriate antibiotics are added to the cement used for both manufacturing the spacer and obtaining fixation based on the antibiogram of the infecting species. The decision on the optimal antibiotic should be made following discussion with a microbiologist. In situations where there is no growth this step is particularly important.
Indications for a static spacer include significant bone loss, ligament laxity in the knee or the deficiency or of the abductor muscles in the hip and significant soft tissue compromise. In all other cases an articulated spacer can be used if available. This has to improve the functional outcomes (10). If soft tissue cover is required in the form of a flap or graft it’s preferable to perform this at the time of the first stage (10). A multidisciplinary approach including a plastic surgeon is paramount in this situation.
Interim period
Antibiotic therapy forms the mainstay during this period and should be directed based on antimicrobial sensitivities. If the organism or sensitivities are not known then empiric therapy should be initiated with the help of a microbiologist until these are known. Every attempt should be made to identify an organism.
The optimal duration of antibiotics is undecided however this should be carried out for 2 to 6 weeks (10,18). The decision for the duration of antibiotics is based on the clinical progress of the patient, wound healing and normalization of the inflammatory markers (ESR and CRP). Once these have normalized antibiotics are discontinued for 4 weeks and inflammatory markers examined weekly. Once these remain normal, the patient is clinically well and the soft tissues have healed the decision is made to proceed to the second stage.
The optimal timing of the second stage is controversial. Some authors have recommended waiting 12 weeks prior to definitive reconstruction (19). Other studies have reported that the re-implantation time can vary from several months to years (20). Extending the interval to the second stage, unless there is a specific reason, does not improve the clinical outcome. Aalirezaie et al. showed through a retrospective study that delaying the second operating time did not improve the infection eradication rate (21). In addition, Vielgut et al. showed that patients who treated between 4 and 11 weeks had a greater success rate than those who were treated before four weeks and after eleven weeks respectively (22).
Regarding the antibiotic therapy it is not necessary to stop 14 days before reimplantation and if dislocation of the spacer occurs it’s not necessary to revise it unless of the risk of skin ulceration or neuro-vascular deficiency or uncontrolled pain (10).
Second stage
The decision of proceeding to perform the definitive reimplantation has to be taken after the resolution of all the signs and symptoms of infection, after the evidence of a decrease of the inflammatory markers (not only the normalization but a descending trend of ESR and CRP) or after a negative needle aspiration (10).
When reimplantation is performed an aggressive debridement of both bone and soft tissues and high-pressure pulsatile lavage are required. Bone preservation in mandatory unless of evidence of signs of infection. This facilitates ease of reconstruction and restoration of a biomechanically stable joint.
Fixation at this stage can be cemented or uncemented. Contemporary antibiotic delivery systems such as DAC (Defensive Antibacterial Coating) can also be used at his stage (23).
If any doubt remains about the presence of infection at this stage a leucocyte esterase strip test and/or a frozen section can be performed looking for 5 to 10 PMNs in each of at least 5 separate high power (400×) microscopic fields (HPF); if any intraoperative mechanical complication occurs or there is evidence of a persistent infection an aggressive debridement followed by a cemented spacer reimplantation (a repeat of the first stage) is required (10).
Results
Knee
The rate of eradication of infection after a two-stage procedure is described in the literature between 54% and 100% with an average of 84.8%. This percentage seems to be higher when using an articulated spacer compared to a static spacer (92.5% vs. 74%). Functional results analyzed using the Knee Society Score range from 63.8 to 86.0 with an average score of 77.8, showing no significant differences from one-stage procedures (ranging from 72 to 88 with an average score of 80) (24).
The use of an articulated spacer seems to lead to a higher infection eradication rate than using a static spacer (91.2% versus 87%) (25).
Hip
Petis et al. describe the re-infection rate at 10% at 1 year, 14% at 5 years, and 15% at 10 and 15 years analyzing results on a group of 164 two-stage revisions (26).
The use of a mobile spacer seems to improve hip function compared to a cemented fixed spacer with no differences in re-infection rate while presenting a minimal increased risk of fracture (2% vs. 0%) (27).
Shoulder
Buchalter et al. describe the results on 19 patients with an average follow-up of 63 months. The reinfection rate was 26% while the American Shoulder and Elbow Surgeons (ASES) Shoulder Assessment score was 69. Patients also showed a significant increase in elevation from 58 to 119 degrees (28).
Conclusions
PJI are very challenging for every surgeon skilled in prosthetic surgery. It’s necessary to make an exact preoperative diagnosis and to treat them with the proper technique.
Evolution can be classified by using four parameters.
- Infection resolution without a continuative antibiotic therapy;
- Infection under control by using an antibiotic suppressive therapy;
- Other surgery to be performed in one of each steps;
- Death within or after one year.
In a lot of cases two-stage revisions of PJI are necessary although they are very invasive, although they can put a strain on the patients clinic and on the biomechanics of the joint affected.
Further studies are needed to establish the perfect timing between the two stages, the duration of the antibiotic therapy and to standardize the diagnostic chart
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Prosthetic Joint Infection”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-84). The series “Prosthetic Joint Infection” was commissioned by the editorial office without any funding or sponsorship. MF and NAS served as the unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455-60. [Crossref] [PubMed]
-
Global Health Observatory (GHO) data from World Health Organization - Cataldo MA, Petrosillo N, Cipriani M, et al. Prosthetic joint infection: recent developments in diagnosis and management. J Infect 2010;61:443-8. [Crossref] [PubMed]
- Pulido L, Ghanem E, Joshi A, et al. Periprosthetic Joint Infection: The Incidence, Timing, and Predisposing Factors. Clin Orthop Relat Res 2008;466:1710-5. [Crossref] [PubMed]
- Gundtoft PH, Overgaard S, Schønheyder HC, et al. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop 2015;86:326-34. [Crossref] [PubMed]
- Rowan FE, Donaldson MJ, Pietrzak JR, et al. The Role of One-Stage Exchange for Prosthetic Joint Infection. Curr Rev Musculoskelet Med 2018;11:370-9. [Crossref] [PubMed]
- Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 2010;18:771-2. [Crossref] [PubMed]
- Shohat N, Buttaro M, Budhiparama N, et al. Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019;34:S325-S327. [Crossref] [PubMed]
- Shohat N, Tan TL, Della Valle CJ, et al. Development and Validation of an Evidence-Based Algorithm for Diagnosing Periprosthetic Joint Infection. J Arthroplasty 2019;34:2730-2736.e1. [Crossref] [PubMed]
- Parvizi J, Gehrke T, Mont MA, et al. Proceedings of the International Consensus Meeting on Prosthetic Joint Infection 2018. J Arthroplasty 2019;34:S1-S2.
- Toms AD, Davidson D, Masri BA, et al. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg (Br) 2006;88:149-55. [Crossref] [PubMed]
- Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. Timing of implant-removal in late acute periprosthetic joint infection: A multicenter observational study. J Infect 2019;79:199-205. [Crossref] [PubMed]
- Kunutsor SK, Whitehouse MR, Blom AW, et al. One- and two-stage surgical revision of peri-prosthetic joint infection of the hip: a pooled individual participant data analysis of 44 cohort studies. Eur J Epidemiol 2018;33:933-46. [Crossref] [PubMed]
- Bialecki J, Bucsi L, Fernando N, et al. Hip and Knee Section, Treatment, One Stage Exchange: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019;34:S421-S426. [Crossref] [PubMed]
- Insall JN, Thompson F, Brause B. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am 1983;65:1087-98. [Crossref] [PubMed]
- Garvin KL, Hanssen A. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am 1995;77:1576-88. [Crossref] [PubMed]
- de Steiger R. Commentary on: Ultrasonic cement removal in cement-in-cement revision total hip arthroplasty. What is the effect on the final cement-in-cement bond? Bone Joint Res 2019;8:253-4. [Crossref] [PubMed]
- Bernard L, Legout L, Zurcher-Pfund L, et al. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J Infect 2010;61:125-32. [Crossref] [PubMed]
- Warth LC, Hadley CJ, Grossman EL. Two-Stage Treatment for Total Knee Arthroplasty Infection Utilizing an Articulating Prefabricated Antibiotic Spacer. J Arthroplasty 2020;35:S57-S62. [Crossref] [PubMed]
- Aalirezaie A, Abolghasemian M, Busato T, et al. Hip and Knee Section, Treatment, Two-Stage Exchange: Proceedings of International Consensus on Orthopedic Infections J Arthroplasty 2019;34:S439-S443. [Crossref] [PubMed]
- Aali Rezaie A, Goswami K, Shohat N, et al. Time to reimplantation: waiting longer confers no added benefit. J Arthroplasty 2018;33:1850-4. [Crossref] [PubMed]
- Vielgut I, Sadoghi P, Wolf M, et al. Twostage revision of prosthetic hip joint infections using antibiotic-loaded cement spacers: when is the best time to perform the second stage? Int Orthop 2015;39:1731-6. [Crossref] [PubMed]
- Zagra L, Gallazzi E, Romanò D, et al. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: results of a comparative series. Int Orthop 2019;43:111-5. [Crossref] [PubMed]
- Pangaud C, Ollivier M, Argenson JN. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev 2019;4:495-502. [Crossref] [PubMed]
- Bonanzinga T, Tanzi G, Iacono F, et al. Periprosthetic knee infection: two stage revision surgery. Acta Biomed 2017;88:114-9. [PubMed]
- Petis SM, Abdel MP, Perry KI, et al. Long-Term Results of a 2-Stage Exchange Protocol for Periprosthetic Joint Infection Following Total Hip Arthroplasty in 164 Hips. J Bone Joint Surg Am 2019;101:74-84. [Crossref] [PubMed]
- Charette RS, Melnic CM. Two-Stage Revision Arthroplasty for the Treatment of Prosthetic Joint Infection Curr Rev Musculoskelet Med 2018;11:332-40. [Crossref] [PubMed]
- Buchalter DB, Mahure SA, Mollon B, et al. Two-stage revision for infected shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:939-47. [Crossref] [PubMed]
Cite this article as: Franceschini M, Pedretti L, Cerbone V, Sandiford NA. Two stage revision: indications, techniques and results. Ann Joint 2022;7:4.


