
A guide to debridement, antibiotics, and implant retention
Introduction
Periprosthetic joint infection (PJI) is a devastating complication associated with hip and knee arthroplasty. Likelihood of successful treatment of PJI is multifactorial with temporal, patient, and implant factors (1-8). While 2-stage revision surgery is often viewed as the gold standard treatment for PJI, “debridement, antibiotics, and implant retention” (DAIR) has been advocated as a viable treatment due to the reduced morbidity and costs, and improved function with equivalent implant longevity when successful (9,10). To perform a DAIR the patient must have a well-fixed implant that was functioning well prior to the infection, a known organism, and good quality soft tissues without a draining sinus (3).
Despite its benefits, DAIR has demonstrated inferior infection eradication when compared to staged revision surgery (1,5). Given these findings, there are a number of relative contraindications to DAIR that include chronic infection, infection with multi-drug resistant organisms, polymicrobial infections, and fungal organisms (5,11).
While the decreased infection eradication rate may be partially attributable to the aforementioned factors, it is important to note that appropriate surgical technique for DAIR is essential to maximizing the likelihood of success (12,13). However, there is difficulty in analyzing the existing literature due to the heterogeneous nature of what is perceived as constituting a DAIR. This review will discuss the technical aspects of DAIR procedure with particular reference to hip and knee arthroplasty. The principles can be applied to any PJI as the basic principles are universally applicable. We will also cover joint specific issues in hip and knee arthroplasty.
General principles
First and foremost, DAIR should not be viewed as a simple “washout” and left to a junior member of the team to be performed out of hours. It should be viewed as a formal revision procedure and performed by an experienced arthroplasty surgeon on a planned surgical list where possible. Furthermore, arthroscopic intervention should not be considered a definitive treatment for PJI as multiple studies have demonstrated that it has a significantly higher rate of failure compared to formal open DAIR (5). In rare circumstances when a patient is in extremis due to sepsis, emergency arthroscopic washout may be used as a temporizing lifesaving measure for a patient that is not medically well enough to undergo a formal DAIR or the relevant experienced surgical team are not available. This will enable stabilization of the patient in order to plan DAIR or formal revision surgery on a planned list.
There are several important surgical principles that are common to both hip and knee DAIR. Adequate exposure, including extensile exposure if needed, should allow for evaluation of the entire prosthetic-bone interface. If the implants are loose or compromised, then DAIR should be abandoned and the surgeon should proceed with 1- or 2- stage revision.
The operative technique has two important aims. To accurately identify the causative organism(s), thus to optimise post-operative antibiotic therapy, and to eradicate the infection. We recommend gaining samples in the first portion of the operation to minimize the chance of contamination through delayed exposure. In practice however, the exposure required to access all parts of the joint forms a large part of the debridement.
A “no-touch” sampling technique should be used to prevent contamination of microbiological samples to ensure appropriate culture directed antibiotics. Each sample is obtained with a new set of clean instruments to minimize risk of contamination (Figure 1). Once samples are obtained, antibiotics can be administered per pre-operative consultation with infectious disease specialists. A total of 5 or 6 paired samples should be sent to microbiology and histology. This is the optimum number that has been shown to obtain the greatest yield in terms of diagnostic accuracy (14).
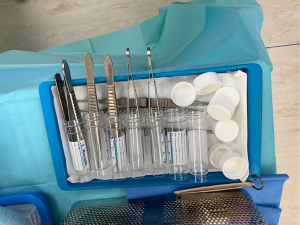
All modular components should be exchanged whenever possible in order to reduce bioburden, gain access to all aspects of well-fixed implants, and allow exposure for a radical synovectomy. Given improved outcomes with modular exchange as opposed to leaving implants in-situ, preoperative planning, including obtaining previous operative reports is necessary to ensure appropriate new modular implants are available prior to intervention (9). Care should also be taken in complex constructs, e.g. hinged knee replacement or constrained acetabular liners, to ensure the equipment is available to disassemble the construct safely without damage to the remaining components.
Generous lavage of the joint cavity is undertaken to further minimize bioburden. In the setting of a DAIR we typically use a minimum of 5 litres of 0.05% chlorhexidine gluconate (CHG) as our irritant as it has been shown to be more effective at reducing biofilm in vitro when compared with saline lavage (15). We also typically utilize a 3-minute minimum dilute 0.35% povidone-Iodine (PI) soak as previously described in the primary setting (17.5 mL of sterile 10% PI for every 500 mL of normal saline lavage) (16).
Absorbable antibiotic loaded calcium phosphate pellets can be used as an additional adjunct allowing local concentrations of antibiotics in the joint cavity significantly higher than the minimum bactericidal concentration (17). Topical delivery of antibiotics from these beads over several weeks potentially lyse remaining bacteria even if protected by remnant biofilm (18). The pellets soften and have not demonstrated significant 3rd body wear (19). The joint capsule can be closed over a deep drain to manage any dead space. We have utilized drains with less frequency given the routine use of tranexamic acid and to minimize loss of local antibiotics eluted from the calcium sulfate beads (20).
Antibiotic coated absorbable suture is utilized deep for closure to minimize colonization of remnant foreign material. Depending on patient factors a closed incision negative pressure wound dressing may also be utilized to aid in wound healing (21).
Broad-spectrum antibiotics are commenced after tissue sampling and continued in consultation with infectious disease specialists.
Knee
The joint is aspirated following skin incision but prior to arthrotomy of the joint capsule to reduce the risk of skin contamination. Care must be taken not to insert the suction catheter into the joint after the arthrotomy is made in order to avoid contamination of samples. Paired samples are systematically taken from the suprapatellar pouch, medial and lateral gutters, posterior capsule, and prosthetic-bone membrane.
A radical synovectomy is then performed of the suprapatellar recess, medial, and lateral gutters (Figure 2). This debridement also allows for improved exposure. Care should be taken to preserve the collateral ligaments if a hinged implant is not in place. The implant-bone interface should be thoroughly debrided to evaluate whether the components are still well-fixed.
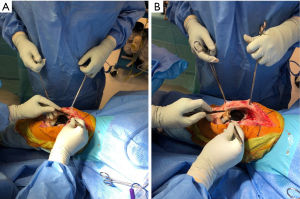
The joint cavity is then irrigated with at least five litres of lavage and the implant surfaces should be scrubbed with a soft nailbrush with CHG to disrupt any biofilm.
While we routinely use CHG pulsatile lavage in the setting of a total knee replacement, in the setting of unicompartmental knee replacement (UKR), the surgeon should consider plain saline wash with gravity flow to avoid potential chemical or mechanical damage to retained cartilage surfaces in other compartments.
After a thorough debridement and lavage the joint cavity is then packed with lap sponges soaked in dilute betadine to prevent undue damage to the femoral and tibial articular surfaces. The surgical incision can either be provisionally closed or covered with a sterile occlusive dressing. The surgeon and staff should then rescrub. The limb should be reprepped and redraped. Clean instruments should be opened.
The cavity is washed with at least three more litres of lavage and any remaining suspect tissue is debrided. Trialing of modular components can be undertaken before selection and implantation of new components. It is then possible to proceed with closure as described previously.
Hip
DAIR should ideally be performed through the same approach that was previously utilized to minimize further contamination of soft tissues and to avoid potential for increased hip instability but only if the surgeon is experienced enough to perform exposure as needed through that particular approach. The hip joint is then aspirated after dissection down to the capsule prior to arthrotomy. Paired samples are then taken from the posterior capsule, anterior capsule and the bone-implant interface of the femoral and acetabular components. The hip is dislocated and the modular femoral head and acetabular liner are removed in order to gain full access to the remaining components. The fifth sample is taken from behind the removed liner (if a modular cementless component is in situ).
A thorough synovectomy is then performed until the entirety of the acetabular and femoral bone-component interfaces can be inspected. It can be difficult to establish whether a cementless component should be kept or a formal revision undertaken as there can be significant proximal membrane with distal fixation. A pragmatic approach is to use a scalpel blade pushed into the implant/bone interface. If solid fixation is encountered the stem can be retained as adequate debridement of the interface can be achieved. If the interface is deeper than the blade length then explantation should be considered.
The trunnion and acetabular shell are then scrubbed with a soft nail brush with CHG to disrupt any possible biofilm. If there is significant trunnion damage or damage to the acetabular shell then DAIR should be abandoned in favor of 1- or 2-stage revision. The joint cavity is then irrigated with at least five liters of lavage followed by a dilute 0.35% PI soak.
After a thorough debridement and lavage the joint cavity is packed with lap sponges soaked in dilute 0.35% PI to maintain the joint space and minimize damage to the trunnion and inner acetabular shell. The surgical incision is then either provisionally closed or covered with a sterile occlusive dressing. The surgeon and staff should then rescrub. The limb should be reprepped and redraped.
Clean instruments should be opened. The cavity is washed with three more liters of lavage and any remaining suspect tissue is debrided. A trial liner and head can then be placed to reassess for hip stability with final implants then seated in place. Larger bearings, dual mobility, or constrained options may need to be considered depending on patient factors and modularity available in the implant system used. We then proceed with closure as described previously.
Conclusions
DAIR is an attractive option in the treatment of PJI but surgical technique is essential to ensuring success both in terms of minimizing bioburden and allowing for accurate microbiology to treat with appropriate antibiotics. The chosen antibiotics should be the best broad-spectrum choice for the relevant population and the common organisms seen in that cohort. Antibiotics are typically narrowed if gram-negative organisms have not been isolated after 48–72 hours. A definitive antibiotic plan including duration can be made after extended cultures are completed. Post-operative oral or intravenous antibiotic therapy is typically for three months. When possible we use oral antibiotics as they have demonstrated similar efficacy in the treatment of PJI and osteomyelitis with significantly increased ease of administration and cost (22). The use of a multidisciplinary team (MDT) can help to further ensure consistent outcomes and has even demonstrated improved infection eradication when utilized for two-stage exchange and should be consulted for all cases of confirmed and suspected PJI (23).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nemandra A Sandiford, Massimo Francescini and Daniel Kendoff) for the series “Prosthetic Joint Infection” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-89). The series “Prosthetic Joint Infection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 2009;63:1264-71. [Crossref] [PubMed]
- Grammatopoulos G, Kendrick B, McNally M, et al. Outcome Following Debridement, Antibiotics, and Implant Retention in Hip Periprosthetic Joint Infection—An 18-Year Experience. J Arthroplasty 2017;32:2248-55. [Crossref] [PubMed]
- Sousa R, Abreu MA. Treatment of Prosthetic Joint Infection with Debridement, Antibiotics and Irrigation with Implant Retention - a Narrative Review. J Bone Jt Infect 2018;3:108-17. [Crossref] [PubMed]
- Haasper C, Buttaro M, Hozack W, et al. Irrigation and Debridement. J Arthroplasty 2014;29:100-3. [Crossref] [PubMed]
- Kunutsor SK, Beswick AD, Whitehouse MR, et al. Debridement, antibiotics and implant retention for periprosthetic joint infections: A systematic review and meta-analysis of treatment outcomes. J Infect 2018;77:479-88. [Crossref] [PubMed]
- Kurtz SM, Lau E, Schmier J, et al. Infection Burden for Hip and Knee Arthroplasty in the United States. J Arthroplasty 2008;23:984-91. [Crossref] [PubMed]
- Kurtz SM, Ong KL, Lau E, et al. Prosthetic Joint Infection Risk after TKA in the Medicare Population. Clin Orthop Relat Res 2010;468:52-6. [Crossref] [PubMed]
- Kurtz SM, Lau E, Watson H, et al. Economic Burden of Periprosthetic Joint Infection in the United States. J Arthroplasty 2012;27:61-5.e1. [Crossref] [PubMed]
- Grammatopoulos G, Bolduc ME, Atkins BL, et al. Functional outcome of debridement, antibiotics and implant retention in periprosthetic joint infection involving the hip: a case–control study. Bone Joint J 2017;99-B:614-22. [Crossref] [PubMed]
- Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br 2004;86:688-91. [Crossref] [PubMed]
- Triantafyllopoulos GK, Soranoglou V, Memtsoudis SG, et al. Implant retention after acute and hematogenous periprosthetic hip and knee infections: Whom, when and how? World J Orthop 2016;7:546. [Crossref] [PubMed]
- Tan TL, Kheir MM, Shohat N, et al. Culture-Negative Periprosthetic Joint Infection: An Update on What to Expect. JB JS Open Access 2018;3:e0060.
- Yoon HK, Cho SH, Lee DY, et al. A Review of the Literature on Culture-Negative Periprosthetic Joint Infection: Epidemiology, Diagnosis and Treatment. Knee Surg Relat Res 2017;29:155-64. [Crossref] [PubMed]
- Atkins BL, Athanasou N, Deeks JJ, et al. Prospective Evaluation of Criteria for Microbiological Diagnosis of Prosthetic-Joint Infection at Revision Arthroplasty. J Clin Microbiol 1998;36:2932-9. [Crossref] [PubMed]
- Schwechter EM, Folk D, Varshney AK, et al. Optimal Irrigation and Debridement of Infected Joint Implants. J Arthroplasty 2011;26:109-13. [Crossref] [PubMed]
- Brown NM, Cipriano CA, Moric M, et al. Dilute Betadine Lavage Before Closure for the Prevention of Acute Postoperative Deep Periprosthetic Joint Infection. J Arthroplasty 2012;27:27-30. [Crossref] [PubMed]
- Howlin RP, Brayford MJ, Webb JS, et al. Antibiotic-Loaded Synthetic Calcium Sulfate Beads for Prevention of Bacterial Colonization and Biofilm Formation in Periprosthetic Infections. Antimicrob Agents Chemother 2015;59:111-20. [Crossref] [PubMed]
- Kallala R, Harris WE, Ibrahim M, et al. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: Safety profile and complication rates. Bone Joint Res 2018;7:570-9. [Crossref] [PubMed]
- Cowie RM, Carbone S, Aiken S, et al. Influence of third-body particles originating from bone void fillers on the wear of ultra-high-molecular-weight polyethylene. Proc Inst Mech Eng H 2016;230:775-83. [Crossref] [PubMed]
- Melvin JS, Stryker LS, Sierra RJ. Tranexamic Acid in Hip and Knee Arthroplasty. J Am Acad Orthop Surg 2015;23:732-40. [Crossref] [PubMed]
- Newman JM, Siqueira MBP, Klika AK, et al. Use of Closed Incisional Negative Pressure Wound Therapy After Revision Total Hip and Knee Arthroplasty in Patients at High Risk for Infection: A Prospective, Randomized Clinical Trial. J Arthroplasty 2019;34:554-9.e1. [Crossref] [PubMed]
- Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med 2019;380:425-36. [Crossref] [PubMed]
- Karczewski D, Winkler T, Renz N, et al. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections: outcome in a centralized and specialized department. Bone Joint J 2019;101-B:132-9. [Crossref] [PubMed]
Cite this article as: Vaz K, Taylor A, Kendrick B, Alvand A. A guide to debridement, antibiotics, and implant retention. Ann Joint 2022;7:5.


