A narrative review of lateral meniscus transplantation with the bridge in slot: technique and outcomes
Introduction
The medial and lateral menisci are critical to normal knee function. They contribute to load sharing, joint lubrication, proprioception, and increase congruity between the femur and tibia (1-3). Up to 70% of the axial load placed on the knee is transmitted through the lateral compartment (4,5). Consequently, lateral meniscus pathology alone causes significant knee dysfunction and contributes to early osteoarthritic changes (6).
Lateral meniscus tears are common in isolation as well as in conjunction with ligamentous injuries such as anterior cruciate ligament (ACL) tears (7). In the setting of complex or irreparable tears, subtotal or total meniscectomy may be required. Lateral meniscus allograft transplantation (LMAT) has been proposed as a technique to spare the degenerative consequences following subtotal or total meniscectomy while also improving clinical outcomes and functionality (8).
There are multiple methods of LMAT, including soft tissue fixation, dovetail, bone plugs, and keyhole techniques. We present the bridge in slot technique. This method allows for secure bone fixation, the ability to efficiently perform concomitant procedures such as osteochondral allograft transplantation or ACL reconstruction, and the advantage of maintaining the relationship between the native posterior and anterior horns of the meniscus (9).
We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-109/rc).
Indications
Ideal candidates for LMAT are patients under 50 years of age with pain localized to the affected compartment. Compartmental pain may follow subtotal or total meniscectomy or an injury creating a functionally meniscectomized knee. When patients present with localized knee pain refractory to physical therapy and other conservative modalities, LMAT may be considered. Pathological findings such as ligamentous instability, chondral defects, and limb malalignment are not contraindications to LMAT but should be addressed with concomitant procedures. These procedures can be performed at the same time or in a staged fashion with the bridge in slot technique.
Contraindications to LMT include obesity, active infection, synovial disease, history of inflammatory arthritis, and frank compartmental arthritic changes (10). The performance of LMAT depends on several factors, including surgeon experience, clinical judgment, and patient preferences.
Preoperative evaluation
History and physical examination
Patient history should include the mechanism of injury, specific symptoms, location and pattern of pain, and surgical history. Patients often report multiple prior procedures, usually including total or subtotal meniscectomy, after a history of knee trauma. They may experience a period of symptomatic improvement, followed by progressive localized knee pain refractory to physical therapy and other conservative measures.
Physical examination should include documentation of muscle atrophy, effusion, swelling, and assessment of range of motion and strength of the affected limb. Joint line tenderness should be specifically assessed. Special tests such as the McMurray and Thessaly tests help assess for existing meniscal pathology. Maneuvers assessing ligamentous stability should be performed concurrently.
Imaging
Radiographic assessment should include anteroposterior (AP), lateral, tunnel, and Merchant views. Standing long limb radiographs can be used to assess for underlying varus or valgus deformities.
Magnetic resonance imaging (MRI) can be used to assess articular cartilage, meniscal tissue, and surrounding ligamentous structures.
Graft sizing and procurement
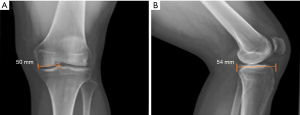
Graft sizing is essential to avoid meniscal allograft size mismatch, graft extrusion, and failure. Radiographic methods described by Pollard et al. are the most widely used (11). On the AP radiograph, the meniscal allograft’s coronal width is calculated by taking the distance from the edge of the ipsilateral tibial spine to the edge of the tibial plateau. On the lateral radiograph, the AP length of the meniscal allograft is calculated by taking the depth of the lateral tibial plateau and multiplying by 0.7 (Figure 1) (12).
Meniscal allografts are obtained within 24 hours of donor death and cryopreserved using dimethyl sulfoxide or freshly frozen by rapid cooling to −80 degrees Celsius. Although rapid cooling may disrupt cell viability, graft biomechanical properties are typically preserved. The risk of disease transmission is lowered by extensive donor screening, graft processing, pulsatile washing, ethanol, and antibiotic cleansing (13).
Surgical technique
Positioning
The procedure can be performed under general, spinal, or regional anesthesia with sedation. The patient is positioned supine with a thigh tourniquet. A leg holder is positioned high on the ipsilateral thigh to allow for knee hyperflexion while the contralateral limb is placed in a well-leg holder with bony prominences well padded. The posterolateral corner should be freely accessible for the inside-out suture technique. Following proper positioning of the patient, routine physical examination of the knee should be performed to check for range of motion and ligamentous stability.
Arthroscopy
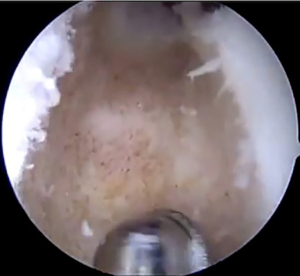
A standard diagnostic arthroscopy is performed using inferolateral and inferomedial portals. The knee is inspected for chondral defects or ligamentous insufficiency. Once this is complete, attention is turned to the preparation of the recipient site. Remnant or residual meniscal tissue is debrided to a 1-to-2 mm peripheral rim until punctate bleeding is encountered, taking care to preserve the capsule’s integrity whenever possible (Figure 2).
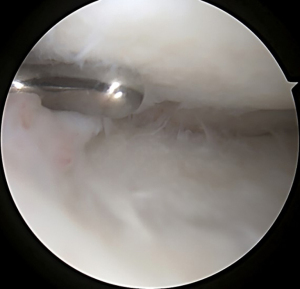
A No. 11 scalpel introduced through the ipsilateral portal under direct visualization can help remove the anterior horn of the meniscus. Preservation of the anterior and posterior horn insertion sites can serve as landmarks during slot preparation. If needed to improve posterior root visualization, a limited notchplasty of the ipsilateral femur can also be performed.
Slot preparation
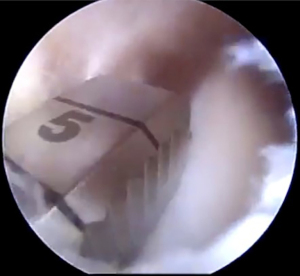
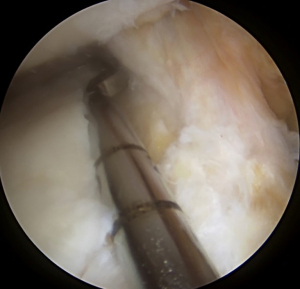
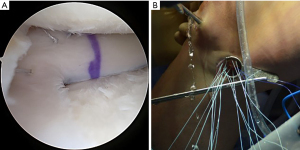
Following spinal needle localization, a small trans-patella tendon arthrotomy is made in line with the anterior and posterior meniscal roots. Electrocautery is then used to mark a line connecting the centers of the residual roots. Next, a 4.5-mm burr is used to make a reference slot along this line, taking care to preserve the native slope of the tibial plateau. The slot’s height and width should match the dimensions of the burr. These dimensions should be confirmed with a depth gauge in the reference slot (Figure 3), after which a guide pin is placed distal and parallel to the bony resection taking care to not penetrate through the posterior cortex of the tibia. The guide pin is then over-reamed with an 8-mm cannulated drill bit, making sure to preserve the posterior cortex. Finally, a box cutter is used to complete an 8 mm × 10 mm reference slot, which can be further refined with a rasp to allow for smooth passage of the graft (Figures 4,5).
Meniscal graft preparation
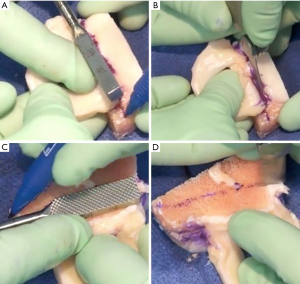
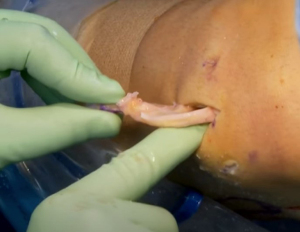
The allograft may be prepared on the back table during the procedure and pre-thawed in normal saline before preparation. The allograft arrives as a hemiplateau with attached meniscal roots. The bony portion will be converted into a rectangular bone bridge undersized by 1 mm to allow easy passage through the tibial slot. Extraneous soft tissue can be removed, and a saw is used to create a bone bridge that is 1 cm in height and 7 mm wide that incorporates the anterior and posterior meniscal root attachments. A marking pen should be used to draw a guideline before saw cuts; the edge of a 7 mm wide rasp can be used to create a straight guideline (Figure 6A-6D). The amount of bone on the graft’s posterior aspect must match the distance from the posterior cortex to the native posterior root. This distance can be estimated intraoperatively with a graduated guide. While the full insertion of the roots is preserved whenever possible, the anterior horn can be as wide 9 mm. In this case, the bone bridge should be cut to match the anterior insertion and then tapered to the target 7 mm size throughout the rest of the bone block. The recipient slot is also widened slightly to accommodate the inconsistency. Finally, a vertical mattress traction stitch with number 0 PDS suture (Ethicon, Blue Ash, OH, USA) is placed at the junction of the meniscus’ posterior and middle thirds (Figure 7).
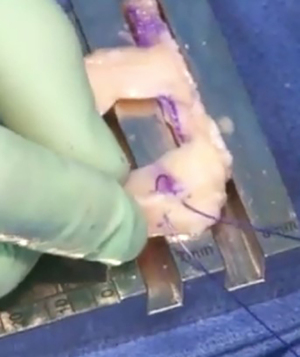
Graft insertion & fixation
A posterolateral incision is made to facilitate graft passage and lateral inside-out meniscocapsular fixation. The 4 cm incision should extend one-third above and two thirds below the lateral joint line to allow for safe suture placement while protecting the neurovascular structures. The superficial interval is developed between the IT band and the biceps femoris, which is then continued between the lateral head of the gastrocnemius muscle and the joint capsule. A Henning retractor is then placed anterior the muscle to protect the neurovascular bundle (Figure 8). Anterior retraction of the IT band will aid in suture passage and allows for tying of the inside-out stitches directly onto the capsule.
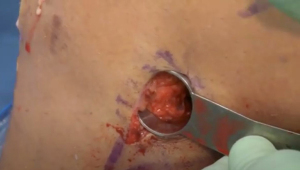
A meniscal repair cannula is placed through the contralateral portal and directed toward the capsular attachment site of the posterior and middle thirds of the meniscus remnant (Figure 9A). A flexible nitinol suture-passing wire is pushed through the posterolateral capsule and retrieved from within the Henning retractor. Next, the pin’s proximal aspect is retrieved through the patella tendon arthrotomy, and the PDS traction sutures are passed through the loop in the nitinol wire (Figure 9B). The pin and the sutures are then withdrawn through the accessory incision. Using the traction suture, the meniscal allograft is pulled into the joint via the anterior arthrotomy while directing the bone bridge into the tibial slot (Figure 10). With the appropriate varus stress, the meniscus can be manually reduced under the condyle with a finger placed through the arthrotomy. The introduction of the graft in hyperflexion, followed by hyperextension with digital pressure, can aid in final reduction. After meniscus reduction, the knee is cycled to seat the graft.
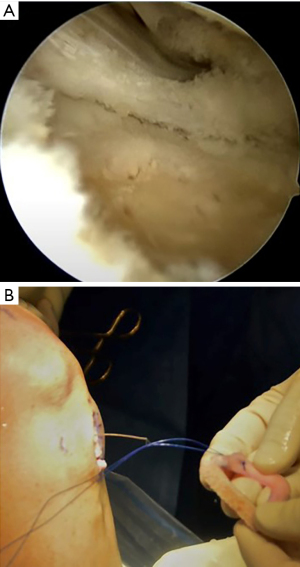
The bone bridge may be secured in the slot with a 7 mm × 23 mm bioabsorbable cortical interference screw. Alternatively, a 4.75 BioComposite SwiveLock anchor (Arthrex Inc, Naples, FL, USA) can be used for easy fixation. Screw or anchor placement is performed in flexion under direct visualization. If a screw is used, the guidewire should be inserted between the graft and the notch, and a tap used to create a pathway for the screw. Manual pressure on the graft using a freer posteriorly and army-navy anteriorly helps avoid graft dislodgement during screw or anchor insertion (Figure 11). Next, the graft is affixed to the capsule with sequentially placed inside-out vertical mattress sutures performed in standard fashion through the accessory incision (Figure 12A). If needed, all-inside and outside-in suture fixation can be used for the posterior and anterior horns, respectively. However, this is often not needed with an appropriately placed accessory window, through which 8–10 sutures can be placed. A combination of superiorly and inferiorly placed vertical mattress stitches on the meniscal allograft helps balance eversion and inversion forces. Careful suture management is necessary to avoid entanglement as sutures are passed through the lateral incision (Figure 12B). Finally, the inside-out stitches are tied down directly onto the capsule. The wounds are copiously irrigated, and incisions are then closed in standard fashion. The limb is immobilized in a hinged knee brace locked in extension.
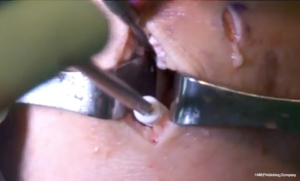
Complications
Meniscus transplantation has a risk profile similar to that of meniscal repair. Complications include infection, incomplete healing, arthrofibrosis, neurovascular damage (peroneal nerve injury laterally), retear, or reoperation. Traumatic tears of the meniscal allograft can be treated with standard arthroscopic meniscal repair, meniscectomy, or even revision MAT. Reoperation rates are high following MAT. However these procedure are typically limited to articular or partial meniscal debridement, often with excellent outcomes (14). Given that MAT is a salvage procedure for patients who have significant knee pathology, the need for future surgeries should not be viewed as a complication. Rather, surgeons must be aware of future procedures and educate their patients to this effect. It should be noted that meniscal damage in the knee is typically progressive and requires multifaceted longitudinal management; MAT is not a cure.
Rehabilitation
Postoperative rehabilitation is of paramount importance for patients undergoing LMAT. Rucinski et al. found that patients who adhered to prescribed rehabilitation protocols were significantly more likely to have positive outcomes following allograft transplantation than those who did not adhere to prescribed protocols (15). Although there is variability within published rehabilitation programs, the senior author prefers one in which patients are restricted to partial weight-bearing for 2 weeks: the knee is locked in extension in a knee brace with periodic gentle range of motion (0° to 90°). Weight-bearing is increased from weeks 3–8, and full weight-bearing and restoration of range of motion are expected by week 8. Running is allowed at 16 weeks, and return to full activity is allowed between 6 and 9 months following surgery (16). Patient-specific factors influence late-rehabilitation outcomes, such as baseline sport/activity levels and long-term goals. Although some surgeons may caution against returning to high-level sporting activity, the rate of return to play following meniscus transplant was found to be 77% in a systematic review published by Hurley and colleagues (17).
Results
Although many studies have scrutinized outcomes following meniscus allograft transplantation, definitive conclusions are difficult to ascertain given the heterogeneity of studies and the lack of randomized trials (16,18-27). The indications for MAT are not standardized, and patient factors such as age, pre-injury activity level, and degree of baseline pathology may impact outcomes. Similarly, MAT’s technical aspects are not uniform. Surgeon experience, the technique employed, and combination with concomitant procedures may influence outcomes. Finally, the outcomes themselves are not standardized, as different studies employ different patient-reported outcome measures (PROMs) and different definitions of treatment failure. Repeat arthroscopy, revision MAT, conversion to arthroplasty, radiographic evidence of graft extrusion, and/or inability to return to sport have all been used as markers of treatment failure (12).
Despite these limitations, readers should be optimistic regarding the outcomes of MAT, given that the majority of studies report positive findings at increasingly distant follow-up. Herein, results from select clinical investigations involving the bridge in slot or bone trough technique are discussed. In a small retrospective case series, Chalmers et al. reported on 13 athletes who underwent MAT using the bridge in slot technique (n=10) or bone plug technique (n=3) at an average follow-up of 3.3 years (19). They reported significant improvements in Knee Injury and Osteoarthritis Outcome Score (KOOS), Lysholm, and International Knee Documentation Committee (IKDC) scores at final follow-up compared to baseline. Ten of the 13 (77%) patients returned to sporting activity within the follow-up period. No differences were reported between those who underwent LMAT (n=10) vs. medial meniscus allograft transplantation (MMAT; n=3). In a case series including 49 patients treated with MAT using either keyhole or bone-trough techniques at an average follow-up of 11.5 years, Kim et al. found significantly improved Lysholm scores at final follow-up compared to preoperative baseline. Furthermore, they reported a 10-year graft survival rate of 98% and a 15-year graft survival rate of 93% (22). Saltzman et al. also reported on outcomes of MAT at long-term follow-up (16). They followed 22 patients who underwent MAT using the bridge in slot technique and found that patients reported significant improvement in all PROMs assessed at final follow-up compared to preoperative scores. They reported no significant differences in outcomes between patients who underwent LMAT (n=9) and MMAT (n=13). Overall, 3 patients experienced failure, as defined by the need for subsequent surgical intervention. Of these 3, one was a medial transplant, and two were lateral. In a study comparing soft-tissue capsulodesis plus sutures through tunnels vs. bone trough LMAT techniques, Masferrer-Pino and colleagues followed 22 patients for an average of 2.1 years (23). Although they found that the capsulodesis group had significantly less meniscus extrusion on follow-up MRI, no differences in PROMs were found at follow-up between the groups. Both groups had significantly improved PROMs compared to preoperative scores.
Several systematic reviews analyzing MAT outcomes have been published (17,28-32). Bin et al. performed a meta-analysis, including 9 studies and 694 total MAT procedures (407 LMAT, 287 MMAT) (28). In the LMAT cohort, 89.2% of grafts survived for 5–10 years, while 56.6% survived for greater than 10 years (28). There was no significant difference in survival rates between LMAT and MMAT. Notably, the authors found that the standardized mean difference in pain scores and weighted mean difference in Lysholm scores were significantly better at minimum 5-year follow-up in the LMAT group than the MMAT group. In another meta-analysis, Lee and colleagues sought to investigate the difference in outcomes for those receiving MAT alone vs. those receiving MAT with other procedures (31). They reported 24 studies with 1882 total MAT cases (1,011 LMAT, 871 MMAT): 53% of the cases were isolated MAT while 47% were combined with other procedures. They included all common techniques of graft fixation. Overall, they found no significant differences in PROMs between those who received isolated vs. combined MAT procedures. They were unable to statistically analyze differences in graft survival rates due to study heterogeneity. However, they reported that 4 studies found no difference in graft survival for isolated vs. combined MAT, and 3 studies found that concomitant procedures were risk factors for graft failure. Another systematic review published by Hergan et al., analyzed 14 studies with 352 total MAT procedures at an average follow-up of 54 months (30). The included studies reported various fixation methods, including bone plugs, bridge in slot, soft tissue, and keyhole techniques. Overall, they found improved PROMs in all studies and no significant differences in PROMs between those receiving LMAT vs. MMAT or those receiving isolated vs. combined procedures. Recently, Fanelli et al. (29) performed a meta-analysis seeking to determine MAT outcome predictors, including 52 studies, 3,460 MAT procedures, and 30 different reported predictors. Overall, they found that patients who underwent LMAT procedures had significantly improved PROMs compared to those receiving MMAT. They also found that fresh-frozen allografts were associated with significantly lower failure rates compared to cryopreserved allografts. Male sex, lower BMI, younger age, and fewer previous ipsilateral knee procedures were all associated with better outcomes for MAT. Notably, on the fixation technique, they found that those receiving grafts with soft-tissue techniques reported significantly better postoperative IKDC scores compared to those undergoing bony fixation techniques. However, no differences between these groups in Lysholm, Tegner, or VAS scores were reported, nor were there significant differences in graft failure rates (30).
Overall, the literature is heterogeneous, and it is difficult to draw definitive conclusions. There is some evidence that those undergoing LMAT have better outcomes than those undergoing MMAT, but these findings have not been consistently reported. Similarly, there is some evidence that isolated procedures perform better than combined procedures, but numerous conflicting studies refute this assertion. In general, studies report improved PROMs at mid to long-term follow-up and relatively low graft failure rates.
Conclusions
The indications, patient assessment, planning, surgical technique, and outcomes of LMAT with the bridge in slot technique have been described in this review. The bridge in slot method is reliable, repeatable, and provides excellent fixation of the graft. Furthermore, it is easily adaptable to accommodate variable patient anatomy and allows for a seamless combination with concomitant procedures. The senior author has used this technique with good clinical results for over 20 years. There is extensive published evidence supporting the use of MAT as a viable treatment option. We suggest that LMAT with the bridge in slot technique should be viewed as an essential tool within the specialist sports medicine orthopaedist’s armamentarium against severe meniscal pathology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Alberto Grassi and Stefano Zaffagnini) for the series “The Lateral Meniscus” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-109/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-109/coif). The series “The Lateral Meniscus” was commissioned by the editorial office without any funding or sponsorship. BJC reports research support, being a paid consultant, and financial or material support from Aesculap; research support from NIH; publishing royalties and financial or material support from Operative Techniques in Sports Medicine; financial or material support from Ossio; financial or material support, being a paid consultant, and research support from Regentis; financial or material support from Smith and Nephew; being a paid consultant, grants, and financial or material support from Arthrex Inc.; IP royalties from Elsevier Publishing; and financial or material support from Bandgrip Inc., financial or material support from Acumed LLC; financial or material support from Encore Medical, LP; financial or material support from GE Healthcare; financial or material support from Merck Sharp & Dohme Corporation; financial or material support from SportsTek Medical, Inc; and financial or material support from Vericel Corporation, all outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haut Donahue TL, Hull ML, Rashid MM, et al. The sensitivity of tibiofemoral contact pressure to the size and shape of the lateral and medial menisci. J Orthop Res 2004;22:807-14. [Crossref] [PubMed]
- Henning CE, Lynch MA. Current concepts of meniscal function and pathology. Clin Sports Med 1985;4:259-65. [Crossref] [PubMed]
- Levy IM, Torzilli PA, Gould JD, et al. The effect of lateral meniscectomy on motion of the knee. J Bone Joint Surg Am 1989;71:401-6. [Crossref] [PubMed]
- Seedhom BB, Hargreaves DJ. Transmission of the Load in the Knee Joint with Special Reference to the Role of the Menisci: Part II: Experimental Results, Discussion and Conclusions. Eng Med 1979;8:220-8. [Crossref]
- Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health 2012;4:340-51. [Crossref] [PubMed]
- Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br 1948;30B:664-70. [Crossref] [PubMed]
- Feucht MJ, Bigdon S, Bode G, et al. Associated tears of the lateral meniscus in anterior cruciate ligament injuries: risk factors for different tear patterns. J Orthop Surg Res 2015;10:34. [Crossref] [PubMed]
- Figueroa F, Figueroa D, Calvo R, et al. Meniscus allograft transplantation: indications, techniques and outcomes. EFORT Open Rev 2019;4:115-20. [Crossref] [PubMed]
- Farr J, Meneghini RM, Cole BJ. Allograft interference screw fixation in meniscus transplantation. Arthroscopy 2004;20:322-7. [Crossref] [PubMed]
- Trentacosta N, Graham WC, Gersoff WK. Meniscal Allograft Transplantation: State of the Art. Sports Med Arthrosc Rev 2016;24:e23-33. [Crossref] [PubMed]
- Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy 1995;11:684-7. [Crossref] [PubMed]
- Frank RM, Cole BJ. Meniscus transplantation. Curr Rev Musculoskelet Med 2015;8:443-50. [Crossref] [PubMed]
- Mickiewicz P, Binkowski M, Bursig H, et al. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank 2014;15:307-17. [Crossref] [PubMed]
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med 2014;42:892-7. [Crossref] [PubMed]
- Rucinski K, Cook JL, Crecelius CR, et al. Effects of Compliance With Procedure-Specific Postoperative Rehabilitation Protocols on Initial Outcomes After Osteochondral and Meniscal Allograft Transplantation in the Knee. Orthop J Sports Med 2019;7:2325967119884291. [Crossref] [PubMed]
- Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg 2012;25:165-75. [Crossref] [PubMed]
- Hurley ET, Davey MS, Jamal MS, et al. High rate of return-to-play following meniscal allograft transplantation. Knee Surg Sports Traumatol Arthrosc 2020;28:3561-8. [Crossref] [PubMed]
- Zhang H, Chen S, Qiu M, et al. Lateral meniscus allograft transplantation with platelet-rich plasma injections: A minimum two-year follow-up study. Knee 2018;25:568-76. [Crossref] [PubMed]
- Chalmers PN, Karas V, Sherman SL, et al. Return to high-level sport after meniscal allograft transplantation. Arthroscopy 2013;29:539-44. [Crossref] [PubMed]
- Cole BJ, Dennis MG, Lee SJ, et al. Prospective evaluation of allograft meniscus transplantation: a minimum 2-year follow-up. Am J Sports Med 2006;34:919-27. [Crossref] [PubMed]
- Kim C, Bin SI, Kim JM, et al. Medial and Lateral Meniscus Allograft Transplantation Showed No Difference With Respect to Graft Survivorship and Clinical Outcomes: A Comparative Analysis With a Minimum 2-Year Follow-Up. Arthroscopy 2020;36:3061-8. [Crossref] [PubMed]
- Kim JM, Bin SI, Lee BS, et al. Long-term Survival Analysis of Meniscus Allograft Transplantation With Bone Fixation. Arthroscopy 2017;33:387-93. [Crossref] [PubMed]
- Masferrer-Pino A, Monllau JC, Ibáñez M, et al. Capsulodesis Versus Bone Trough Technique in Lateral Meniscal Allograft Transplantation: Graft Extrusion and Functional Results. Arthroscopy 2018;34:1879-88. [Crossref] [PubMed]
- Noyes FR, Barber-Westin SD. Long-term Survivorship and Function of Meniscus Transplantation. Am J Sports Med 2016;44:2330-8. [Crossref] [PubMed]
- Rue JP, Yanke AB, Busam ML, et al. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med 2008;36:1770-8. [Crossref] [PubMed]
- Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc 2006;14:694-706. [Crossref] [PubMed]
- Yanke AB, Chalmers PN, Frank RM, et al. Clinical outcome of revision meniscal allograft transplantation: minimum 2-year follow-up. Arthroscopy 2014;30:1602-8. [Crossref] [PubMed]
- Bin SI, Nha KW, Cheong JY, et al. Midterm and Long-term Results of Medial Versus Lateral Meniscal Allograft Transplantation: A Meta-analysis. Am J Sports Med 2018;46:1243-50. [Crossref] [PubMed]
- Fanelli D, Mercurio M, Gasparini G, et al. Predictors of Meniscal Allograft Transplantation Outcome: A Systematic Review. J Knee Surg 2021;34:303-21. [Crossref] [PubMed]
- Hergan D, Thut D, Sherman O, et al. Meniscal allograft transplantation. Arthroscopy 2011;27:101-12. [Crossref] [PubMed]
- Lee BS, Kim HJ, Lee CR, et al. Clinical Outcomes of Meniscal Allograft Transplantation With or Without Other Procedures: A Systematic Review and Meta-analysis. Am J Sports Med 2018;46:3047-56. [Crossref] [PubMed]
- Smith NA, Parkinson B, Hutchinson CE, et al. Is meniscal allograft transplantation chondroprotective? A systematic review of radiological outcomes. Knee Surg Sports Traumatol Arthrosc 2016;24:2923-35. [Crossref] [PubMed]
Cite this article as: Nathan LI, Kester BS, Condron NB, Evuarherhe A Jr, Cole BJ. A narrative review of lateral meniscus transplantation with the bridge in slot: technique and outcomes. Ann Joint 2022;7:17.