
Lateral collagen meniscus implant (CMI): techniques and outcomes—a narrative review
Introduction
The comprehension of the anatomy and biomechanics of the joint is mandatory to understand that the clinical decision to perform a meniscectomy or a meniscal suture could have catastrophic consequences at a long-term follow-up. Moreover, the medial and the lateral compartment of the knee have different kinematic properties and the clinician must take into account the different degree of mobility, bony structure and load distribution between these two compartments.
Biomechanical studies have demonstrated the essential role of the menisci on load transfer. A total meniscectomy reduces the contact area by 33 to 50 percent in a fully extended knee (1).
Moreover, Walker et al. showed that the lateral compartment is much more dependent on the meniscus function than the medial one. The lateral meniscus carries a higher percentage of load transfer than the medial meniscus because in the medial compartment, a higher load is transferred directly by the exposed cartilage surface (2). This could be explained by the different bony morphology of the tibiofemoral compartments: in the sagittal plane, in the medial side the convexity of the femoral condyle and the concavity of the medial tibial plateau give some degree of congruity, even after a meniscectomy. On the contrary, on the lateral side, both the convexity of the femoral condyle and the lateral tibial plateau make this compartment much more prone to an increase in peak contact pressures after meniscus resection (3).
From a clinical point of view, this aspect has been confirmed by worse results reported after lateral meniscectomy rather than the medial meniscectomy at a long-term follow-up (4,5). These findings are guaranteed if the meniscectomy is performed during adolescence: in a prospective 30 years of follow-up study, after medial meniscectomy about 80% maintained good or excellent clinical results, while if lateral meniscectomy was performed these results dropped to less than 50% (6) (Figure 1).
Despite these clear evidences, the number of meniscus surgeries performed in Europe and the United States is increasing every year due to a more active and older population (7-9). Even if the percentages of meniscus repair procedures are increasing, meniscectomy is still the most performed meniscal treatment.
In fact, most of the meniscal lesions are in the white-white zone or, especially in older patients, could involve degenerated tissue and could have a complex pattern. Moreover, complex dislocated bucket handle tear could be difficult to reduce and suture (10).
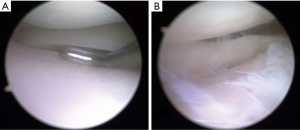
A subgroup of patients experience pain and worsening of symptoms due to the increased contact stress within the joint over the course of months or years after, a finding referred to as “post-meniscectomy syndrome” (11) (Figure 2). If these patients do not have advanced osteoarthritis (OA), meniscal replacement surgery should be considered (12). The current treatment options include a meniscus allograft transplantation or meniscal scaffold implant based on the degree of the previous meniscectomy (13). There are three different scaffold types described in the literature: the collagen meniscus implant (CMI) derived from a bovine collagen, the Actifit, a polyurethane scaffold, and the 3D printed scaffolds (14,15). While the latter solution has been recently proposed as an experimental treatment and only a few case reports are available, CMI and Actifit have been widely studied.
This article aims to provide an overview regarding the current indications, surgical technique, and outcomes of the lateral CMI, the first meniscal scaffold developed.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-21-2/rc).
Methods
A research on PubMed
CMI—basic science
Since the accepted indication for meniscus allograft transplantation is a prior total or subtotal meniscectomy, an allograft is not a solution for the treatment of partial meniscus defect. Moreover, the limited availability of meniscus allograft, the need for a precise sizing before the surgery, and the potential disease transmission motivated some researchers to develop meniscus scaffold for the regeneration of partial meniscus defect.
The CMI (Ivy Sports Medicine, Germany) is a porous biologic scaffold. It is composed of about 97% of collagen type I purified from the bovine Achilles tendon. The remaining portion of the CMI is composed of glycosaminoglycan (GAG).
The size of the scaffold’s micropores have been specifically studied to increase the fibrocartilage maturation while avoiding pseudo-capsule formation and foreign body reaction (16). Moreover, the scaffold has been demonstrated to be safe in terms of cytotoxicity and carcinogenicity.
The first studies on animals were performed by Stone et al.: they demonstrated that a copolymeric collagen-based scaffold can be constructed that is compatible with meniscal fibrochondrocyte growth in vitro and in vivo, that does not inhibit meniscal regeneration in an immature pig, and that may induce regeneration of the meniscus in the mature dog (17). Moreover, animal models demonstrated no evidence of cartilage wear or damage and no immunological reaction (18).
Histological analysis performed on both animals and humans also showed the healing of the implant with progressive reabsorption of the collagen fibrils within 6 to 18 months and an increased host tissue invasion and vascularization with the final evidence of meniscus-like tissue (19,20).
Indication for surgery
Patients evaluation
Obtaining an accurate history of knee trauma and surgical procedures is mandatory during the initial evaluation of the patients. A history of previous meniscectomy with recurrent knee pain, swelling and mechanical symptoms that worsen during light or moderate physical activity is typically consistent with a post-meniscectomy syndrome. The evidence of bone marrow edema on MRI, further confirm this diagnosis.
Height, weight and body mass index (BMI) should be recorded because the morbidly obese could have less symptom relief from meniscal replacement surgery. With the patient standing, the lower limb alignment must be evaluated; the presence of varus or valgus thrust should also be reported. Then, the physical examination must turn to ligament stability: varo-valgus laxity, as well as anterior and posterior drawer exam and the pivot shift test, must be performed. The presence of anterior cruciate ligament (ACL) laxity is not a strict contraindication for this type of surgery. However, the clinician must consider an associated ACL reconstruction concomitant to the CMI implantation.
A full radiological evaluation must be performed before turning to surgery. It is mandatory to obtain weight-bearing X-rays of the whole limb to asses joint space narrowing, sign of osteonecrosis, advanced OA and measure the mechanical axis. While the evidence of osteonecrosis and OA grade 4 represents a contraindication for surgery, if the patients present a valgus deformity of 5 degrees or more a corrective osteotomy should be planned concomitantly or a staged procedure performed.
An MRI is also essential to confirm the indications for surgery. First of all, the percentage of meniscus previously resected must be evaluated because the CMI should not be implanted in cases of total or near-total meniscectomy. Attention must then be turned to the anterior and posterior horns of the meniscus, which must be intact. Ligamentous injuries such as ACL tear must be evaluated as well because they could modify the surgical planning. Finally, the attention should be turned to the cartilage status and the presence of subchondral bone marrow. If the latter condition is present, there is also a radiological confirmation of the “post-meniscectomy syndrome”. While the cartilage status of the compartment could be useful to establish patients expectations and evidence of bone-bone contact could be an exclusion criteria for the surgery, the presence of a focal lesion is not a contraindication for surgery. Instead, a cartilage restoration procedure (such as microfracture or mosaicplasty) could be indicated in these patients.
The indications for surgery for lateral CMI implantation consist of:
- Irreparable acute lateral meniscal tears requiring partial meniscectomy (acute pattern) or prior traumatic or degenerative loss of lateral meniscal tissue (chronic pattern) greater than 25%;
- Intact anterior and posterior horn attachments of the lateral meniscus;
- Intact rim (1 mm or greater) over the entire circumference of the involved meniscus (a deficient popliteal hiatus can be allowed because, the native meniscal rim has low vascularization and consequently low healing power at this location);
- Diagnosis of Outerbridge grade I to III OA. In the presence of a focal cartilage defects, a cartilage procedure such as microfracture, mosaicplasty or a cartilage scaffold implantation could be performed as an associated surgery;
- ACL deficiency could be present; however, an ACL reconstruction should be performed as a concomitant procedure;
- Valgus alignment of the knee of less than 5°. If the valgus exceeds this threshold, a distal femur osteotomy (DFO) must be performed as a concomitant procedure.
The contraindications for surgery are the following:
- Concomitant posterior cruciate ligament insufficiency of the involved knee;
- Diagnosis of Outerbridge grade IV in the affected joint;
- Uncorrected malformations or axial malalignment greater than 5°;
- Documented allergy to collagen or chondroitin sulfate of animal origin;
- Systemic or local infection;
- History of an anaphylactic reaction;
- Systemic administration of corticosteroid or immunosuppressive agents within 30 days of surgery;
- Evidence of osteonecrosis in the involved knee;
- History of rheumatoid arthritis, inflammatory arthritis, or autoimmune diseases;
- Neurological abnormalities or conditions that would preclude the patient’s requirements for the rehabilitation program;
- Pregnancy;
- A BMI over 30 is considered a relative contraindication (21).
Surgical technique
The technique for arthroscopic lateral CMI implantation is clearly described in the literature.
The patient is positioned supine with a tourniquet and the knee flexed at 90°. A leg holder is placed 5 cm proximal to the superior pole of the patella in order to allow complete visualization of the medial compartment. The opening of the lateral compartment could be achieved by flexing the leg in the “figure of four” position. If the lateral compartment is particularly tight, the second surgeon could provide additional stress by internally rotating the leg and pushing against the medial part of the proximal tibia.
After the positioning of the patient, standard anteromedial and anterolateral are made. Then a standard diagnostic arthroscopy is performed. The ACL should be functionally intact or it should be reconstructed during the same procedure; the degree of cartilage degeneration in each compartment must be assessed as well. In the presence of focal cartilage lesions, these could be treated with standard cartilage procedures such as microfracture, mosaicplasty or scaffold implants.
Then attention should be turned to the lateral compartment. All the unstable and degenerated meniscal tissue should be debrided to a stable rim, unless an acute irreparable tear or the sequelae of a previous meniscectomy are noted. This procedure could be done with a basket or a shaver based on the surgeon preference. It is important to ensure that the anterior and the posterior horn must both be present and functionally stable.
The area of the CMI implantation must be trimmed to a clean border to accept the scaffold easily; the prepared defect size should maintain uniform width of the meniscus rim and extend into the red/white or red/red zone. Additionally, the blood supply from the capsule could be enhanced by making puncture holes in the peripheral rim with a Steadman awl.
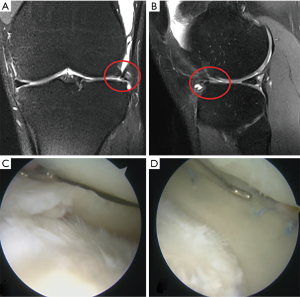
Once the defect area is prepared, the defect size should be measured with the appropriate instrumentation through the ipsilateral portal (Figure 3). Note that the lateral CMI should be oversized by 10% to obtain primary press-fit stability that could help during the following surgical steps. If the popliteal hiatus is included in meniscectomized area, an additional oversizing by 20% could be indicated in order to prevent an excessive movement of the scaffold into the hiatus during the procedure. Once the correct length of the CMI has been estimated, the scaffold could be trimmed using a scalpel. The CMI is then introduced inside the joint with a delivery clamp through an enlarged lateral portal and a blunt probe could be used in order to reach the correct position.
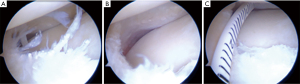
Once a good press-fit stability has been achieved, the scaffold could be sutured to the host meniscus remnant and to the capsule with “all-inside” stitches (Figure 4).
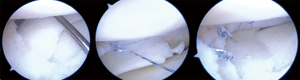
Please note that suturing should begin at the posterior aspect of the CMI to achieve better visualization of the position of the scaffold and prevent intraoperative dislocation. It is also mandatory that the anterior and posterior ends of the CMI are sutured with horizontal sutures, while vertical mattress sutures are used throughout the remained CMI every 5–7 mm (21). During the whole procedure, the suture should not be overtightened because this could damage the CMI structure and lead to vertical CMI tear or instability of the implant. Finally, the scaffold stability must be tested with a probe.
Rehabilitation protocol
The following rehabilitation protocol must be applied after the surgery to avoid early dislocation or failure of the sutures and the implant. A knee brace is applied, locked in full extension and maintained for 6 weeks. Continuous passive motion (CPM) exercises should be performed 4 times per day, up to 60° for the first 2 weeks and up to 90° for the 2nd to 4th week; complete range of motion (ROM) is permitted at the 6th week. Progressive weight-bearing is allowed 3 weeks after surgery with about 30% of the body weight. A progressive increase of the weight bearing should be encouraged from the 4th week to reach the total weight-bearing 6 weeks after the surgery. Muscle-strengthening should start on the second postoperative day with isometric exercises; cycling is allowed at the 4th postoperative week. Full unrestricted activity as tolerated was permitted after 6 months from surgery (22).
Outcomes
The outcomes after the medial CMI have been extensively studied since the medial scaffold was made commercially available about 10 years before the lateral one. For this reason, the long-term results of the CMI are available only for the medial procedure (22). A milestone in the literature is the randomized controlled trial of medial CMI versus medial meniscectomy performed by Rodkey et al. in 2008 (20). The authors reported better clinical scores and a lower reoperation rate in the group of patients treated because of a chronic meniscal deficiency. On the contrary, no clinical differences were reported at 5 years of follow-up if the CMI was implanted in the acute group as a prophylactic procedure.
As already highlighted, the outcomes of the lateral CMI have been investigated by a lower number of trials with a shorter follow-up; a recent systematic review that investigated 396 CMI found that only 10% of them were implanted in the lateral compartment (23).
Hirschmann et al. (24) investigated a series of 67 patients that underwent medial or lateral CMI implantation associated with ACL reconstruction (45%), high tibial osteotomy (7.5%) or microfracture (4.5%). One year of follow-up demonstrated a marked decrease of pain and an improvement in the Tegner, IKDC and Lysholm score. Moreover, the results of the medial and the lateral group were comparable.
In a recent multicenter study, Zaffagnini et al. (21) reported the outcomes of 43 patients clinically evaluated 24±1.9 months after lateral CMI implant. In this study the Lysholm score improved from 64.3±18.4 preoperatively to 93.2±7.2 at final follow-up. Similarly, the pain experienced both during strenuous activity and at rest was significantly reduced. Interestingly, the clinical scores improved from 6 months after surgery to 12 months of follow-up, thus demonstrating a better knee function after the CMI maturation. At 2 years of follow-up, about 60% of patients reported activity levels similar to their preinjury values and the satisfaction rate was 95%. Interestingly, the presence of a higher BMI, the need for concomitant procedures, and a chronic injury pattern resulted in reduced outcomes.
Therefore, this conclusion must be taken into account during the patient’s selection and could help set patient expectations accordingly. Also, the safety of the device was confirmed: adverse events of the scaffold leading to CMI explantation were reported only in 6% of the patients.
In another study, the same author investigated a cohort of 24 patients that underwent lateral CMI for acute irreparable meniscal tears (7 patients) or for a chronic lateral meniscectomy (17 patients). At a minimum 2 year of follow-up, all the clinical scores investigated significantly improved from preoperative evaluation to final follow-up. Moreover, the MRI evaluation demonstrated no progression of cartilage damage; 87.5% of implants were reduced in size, and in 3 cases (12.5%), they were completely resorbed (25).
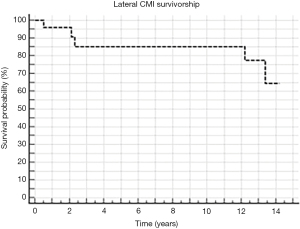
Grassi et al. (unpublished data) investigated the long-term results of the lateral CMI in 19 patients at 12.4 years of mean follow-up. Their results showed that the scaffold provided good long-term results, with a 10-year survival rate of 85% and a 14-year survival rate of 64% (Figure 5). Additionally, 58% of the patients were rated as “good” or “excellent” according to the Lysholm score. Finally, 78% were satisfied by the procedure, even though a general decrease of the clinical scores was reported from the 2-year timepoint with respect to the long-term follow-up. Specifically, the Lysholm score decreased to 82±14 at the final follow-up and the visual analog scale (VAS) for pain was reduced to 3.1±3.1 points at 12 years. Moreover, only 3/19 patients (16%) reported to be completely without pain. Notably, all the average clinical scores were significantly higher compared with the pre-operative status, except for the Tegner score.
Conclusions
The lateral CMI represents an attractive surgical option for treating a “post-meniscectomy syndrome” following a partial arthroscopic meniscal resection. In-vitro and in-vivo studies demonstrated the progressive reabsorption of the CMI and the substitution with a meniscus-like tissue with a potential chondroprotective effect. Satisfactory clinical results have been reported in the vast majority of patients at a short-term follow-up, however, patient selection and the treatment of concomitant knee pathology is mandatory in order to achieve a clinical improvement. Additional long-term studies are needed to evaluate possible cartilaginous protection and could help to better identify the long-term benefits and failures of the procedure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “The Lateral Meniscus”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-21-2/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-21-2/coif). The series “The Lateral Meniscus” was commissioned by the editorial office without any funding or sponsorship. SZ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from March 2021 to February 2023. AG served as the unpaid Guest Editor of the series. SZ receives the consulting fees from Depuy-Synthes and Smith and Nephew, and receives payments from Depuy-Synthes and Smith and Nephew). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang AL, Miller SL, Coughlin DG, et al. Tibiofemoral contact pressures in radial tears of the meniscus treated with all-inside repair, inside-out repair and partial meniscectomy. Knee 2015;22:400-4. [Crossref] [PubMed]
- Walker PS, Hajek JV. The load-bearing area in the knee joint. J Biomech 1972;5:581-9. [Crossref] [PubMed]
- McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br 2006;88:1549-56. [Crossref] [PubMed]
- Jørgensen U, Sonne-Holm S, Lauridsen F, et al. Long-term follow-up of meniscectomy in athletes. A prospective longitudinal study. J Bone Joint Surg Br 1987;69:80-3. [Crossref] [PubMed]
- Hede A, Larsen E, Sandberg H. The long term outcome of open total and partial meniscectomy related to the quantity and site of the meniscus removed. Int Orthop 1992;16:122-5. [Crossref] [PubMed]
- McNicholas MJ, Rowley DI, McGurty D, et al. Total meniscectomy in adolescence. A thirty-year follow-up. J Bone Joint Surg Br 2000;82:217-21. [Crossref] [PubMed]
- Herzog MM, Marshall SW, Lund JL, et al. Trends in Incidence of ACL Reconstruction and Concomitant Procedures Among Commercially Insured Individuals in the United States, 2002-2014. Sports Health 2018;10:523-31. [Crossref] [PubMed]
- Jacquet C, Pujol N, Pauly V, et al. Analysis of the trends in arthroscopic meniscectomy and meniscus repair procedures in France from 2005 to 2017. Orthop Traumatol Surg Res 2019;105:677-82. [Crossref] [PubMed]
- Kawata M, Sasabuchi Y, Taketomi S, et al. Annual trends in arthroscopic meniscus surgery: Analysis of a national database in Japan. PloS One 2018;13:e0194854. [Crossref] [PubMed]
- Hulet C, Pereira H, Peretti G, et al. editors. Surgery of the Meniscus. 1st edition. Springer-Verlag Berlin Heidelberg, 2016.
- Drobnič M, Ercin E, Gamelas J, et al. Treatment options for the symptomatic post-meniscectomy knee. Knee Surg Sports Traumatol Arthrosc 2019;27:1817-24. [Crossref] [PubMed]
- Getgood A, LaPrade RF, Verdonk P, et al. International Meniscus Reconstruction Experts Forum (IMREF) 2015 Consensus Statement on the Practice of Meniscal Allograft Transplantation. Am J Sports Med 2017;45:1195-205. [Crossref] [PubMed]
- Filardo G, Andriolo L, Kon E, et al. Meniscal scaffolds: results and indications. A systematic literature review. Int Orthop 2015;39:35-46. [Crossref] [PubMed]
- Filardo G, Petretta M, Cavallo C, et al. Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold. Bone Joint Res 2019;8:101-6. [Crossref] [PubMed]
- Ghodbane SA, Brzezinski A, Patel JM, et al. Partial Meniscus Replacement with a Collagen-Hyaluronan Infused Three-Dimensional Printed Polymeric Scaffold. Tissue Eng Part A 2019;25:379-89. [Crossref] [PubMed]
- Klompmaker J, Jansen HW, Veth RP, et al. Porous implants for knee joint meniscus reconstruction: a preliminary study on the role of pore sizes in ingrowth and differentiation of fibrocartilage. Clin Mater 1993;14:1-11. [Crossref] [PubMed]
- Stone KR, Rodkey WG, Webber R, et al. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med 1992;20:104-11. [Crossref] [PubMed]
- Stone KR, Steadman JR, Rodkey WG, et al. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am 1997;79:1770-7. [Crossref] [PubMed]
- Rodkey WG, Steadman JR, Li ST. A clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop Relat Res 1999;S281-92. [Crossref] [PubMed]
- Rodkey WG, DeHaven KE, Montgomery WH, et al. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am 2008;90:1413-26. [Crossref] [PubMed]
- Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Two-Year Clinical Results of Lateral Collagen Meniscus Implant: A Multicenter Study. Arthroscopy 2015;31:1269-78. [Crossref] [PubMed]
- Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med 2011;39:977-85. [Crossref] [PubMed]
- Grassi A, Zaffagnini S, Marcheggiani Muccioli GM, et al. Clinical outcomes and complications of a collagen meniscus implant: a systematic review. Int Orthop 2014;38:1945-53. [Crossref] [PubMed]
- Hirschmann MT, Keller L, Hirschmann A, et al. One-year clinical and MR imaging outcome after partial meniscal replacement in stabilized knees using a collagen meniscus implant. Knee Surg Sports Traumatol Arthrosc 2013;21:740-7. [Crossref] [PubMed]
- Zaffagnini S, Marcheggiani Muccioli GM, Bulgheroni P, et al. Arthroscopic collagen meniscus implantation for partial lateral meniscal defects: a 2-year minimum follow-up study. Am J Sports Med 2012;40:2281-8. [Crossref] [PubMed]
Cite this article as: Grassi A, Lucidi GA, Agostinone P, di Paolo S, dal Fabbro G, Zaffagnini S. Lateral collagen meniscus implant (CMI): techniques and outcomes—a narrative review. Ann Joint 2022;7:18.


