
Narrative review of the epidemiology, economic burden, and societal impact of metastatic bone disease
Introduction
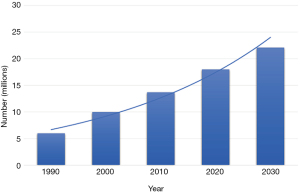
Advances in the early detection and treatment of cancer, together with a rapidly growing and aging population, have fueled the increase in the prevalence of cancer. More than 60% of cancer patients are greater than the age of 65 (1) and the U.S. Bureau of Census projects that the population aged 65 years and older is expected to increase from 56 million in 2020 to 86 million in 2050 (2). More than 17 million Americans living today have a history of cancer and an additional 1.8 million new cancer cases are expected to be diagnosed in 2020 (3,4). By January 1, 2030, it is estimated that the population of people with a history of cancer will increase to more than 22.1 million (Figure 1) (4). As patients with cancer live longer, the incidence of metastatic bone disease (MBD) is also increasing, however accurate figures are not readily available for how many of these patients will develop skeletal metastases. The management of patients with MBD is complex and requires the utilization of various resources. Here, we review the epidemiology of MBD and the profound effects it has on patients, caregivers, society and the economy. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-97/rc).
Methods
MEDLINE, BioMed Central, Gale Onefile: Health and Medicine, Access Medicine, Access Surgery and Cochrane Library were searched using the terms ‘metastatic bone disease’, ‘skeletal metastases’, ‘economic burden’, ‘societal impact’, ‘epidemiology’ and ‘quality of life’ from 1976 through May 2020. Papers were selected according to their relevance to the focus of this article. Papers that were not pertinent to the focused purpose of this review or those that were in a language other than English were excluded.
Epidemiology of metastatic bone disease
Bone is the third most common organ affected by metastasis, after the lungs and liver (5). While skeletal metastases can occur in almost any malignancy, the most common cancers that spread to bone are prostate, breast, lung, kidney and thyroid. Among these malignancies, breast, prostate and lung have the highest incidence in the US (Table 1) (3), and represent roughly 70% of the cases of metastatic bone disease (MBD) seen by clinicians (6). Population-based estimates of the prevalence of patients with bone metastases secondary to solid tumors in the US are limited. Nonetheless, recent literature estimates the prevalence of MBD in the U.S. in a given year to be somewhere between 300,000 and 600,000 cases (5-9). Roughly, 5–6% of breast cancer patients present with MBD at diagnosis (10) and among those who present without metastatic disease, the risk of developing skeletal metastases increases at 5, 10 and 15 years (6.5%, 10.5% and 12% respectively) (11). In addition to breast cancer, a significant portion of patients with advanced prostate, lung, thyroid, renal and bladder carcinoma also have MBD (Table 1) (12). These numbers, however, likely underestimate MBD, as autopsy studies may suggest that the incidence of bone metastases in patients who die of cancer is close to 70% (13). Additionally, improved screening methods and advanced treatments have subsequently led to both more accurate detection and improved survival, therefore increasing the incidence of MBD.
Table 1
| Cancer type | Estimated number of new cases in the US, 2020 | Incidence of MBD in patients with advanced disease (%) |
|---|---|---|
| Breast | 279,100 | 65–75 |
| Prostate | 191,930 | 65–75 |
| Lung | 228,820 | 30–40 |
| Renal | 73,750 | 40 |
| Thyroid | 52,890 | 20–25 |
| Bladder | 81,400 | 60 |
| Melanoma | 100,350 | 14–45 |
MBD, metastatic bone disease.
The most common sites of bone metastases include the spine, pelvis, ribs, skull, proximal humerus and the femur (14). Metastatic bone lesions alter the structural integrity of the bone, leading to an increase in the risk for skeletal-related events (SREs) such as pathologic fracture, spinal cord compression, hypercalcemia of malignancy, and severe bone pain requiring palliative radiotherapy or surgery (15). Tumors that tend to produce radiolucent (lytic) lesions have a higher fracture rate than those that produce radiodense (sclerotic) lesions (14,16).
The incidence of SRE in MBD is high. In a study of patients with newly diagnosed MBD, SREs were present in 22% of the patients at diagnosis of bone disease. Of those not presenting with an SRE at diagnosis, 47% of lung cancer patients, 46% of prostate cancer and 52% of breast cancer patients experienced an SRE during the follow-up period (17). Similarly, in another long term study, the cumulative incidences of SREs were 47%, 31.4% and 38% in breast cancer, prostate cancer and multiple myeloma, respectively (18). Furthermore, patients with MBD often experience multiple SREs, that typically occur at a more rapid rate following the initial event (15,18).
SREs represent a substantial challenge in the management of MBD as among other issues, they are associated with a lower survival rate (15,19-21). Survival varies following a SRE. Longer mean survivals are seen in thyroid (26 months), breast (19 months), and prostate cancer (18 months), whereas poor mean survivals are a feature of lung cancer (6 months) and cancer of unknown primary (16). In a study from the Scandinavian Sarcoma Group on 1195 surgically treated non-spinal metastases, the 1-year survival was 41%, whereas the 5-year survival was 2%. The longest median survival was in myeloma patients (26.3 months), followed by thyroid cancer (22.7 months), breast cancer (12 months), and kidney cancer (10 months) patients. Melanoma had the worst prognosis (2.3 months) (22). The occurrence of a pathological fracture is directly related to a decreased survival. In a study of 3,049 patients with MBD, pathologic fractures had up to a 32% increased risk of death compared to the absence of a pathologic fracture (20).
Generally, and with the exception of selected cases with single metastases from renal or breast cancer, the diagnosis of MBD signifies that the disease is incurable. However, especially with advancements in the field of oncology, patients with MBD may survive for an extended period of time. Subsequently, they are at risk of developing SREs. They pose a significant clinical concern and delaying or preventing them is an important treatment objective.
Economic burden
As the number of cancer survivors have continued to rise in the past two decades, the health care expenditures on cancer treatment have also greatly increased. According to the Agency for Healthcare Research and Quality, a total of $57 billion was spent on cancer care in 2001, compared with $88.3 billion in 2011 (23), and the National Cancer Institute reported that national estimates of cancer care costs are expected to rise to as high as between $173 to $207 billion in 2020 (24). These costs are only the direct medical costs of cancer treatment. Direct medical costs are those associated with services that patients receive, including hospitalizations, surgery, physician visits, radiation therapy and chemotherapy/immunotherapy (25). The economic burden of cancer, however, is much greater than just the direct healthcare cost, and also includes indirect costs. These are the monetary losses associated with time spent receiving medical care, time lost from work or other usual activities (morbidity costs), and lost productivity due to premature death (mortality costs). These costs are borne by patients, caregivers, families, employers and society as a whole (16). In a projection from the NIH, the indirect costs are estimated to increase from approximately $116 billion in 2000 to $148 billion in 2020 (25). The direct medical costs associated with cancer vary significantly by cancer site and the phase of treatment. Based on a report from the NIH in 2010, in the initial phase of care, the annualized mean net costs of care were $66,586 for lung cancer, $25,386 for breast and $21,681 for prostate cancer. The mean annual costs during the continuing phase of care were $8,130, $2,207 and $3,201 respectively. Among the patients who died of cancer, the mean annual costs of treatment during the last year of life were $115,655, $78,570, and $77,803 (24). MBD is a significant driver of this cost, as nearly one-fifth of the total oncology cost is due to MBD (8). The annual cost of care directly attributable to skeletal metastases is estimated to be about $18,272 per patient (16). The mean direct medical cost of patients with MBD (all cancers) is roughly $75,329, when compared with $31,382 in controls (patients with cancer but without MBD) (8). The treatment cost of skeletal metastases is exceedingly high, especially in the case of SREs. They are associated with a significant consumption of healthcare resources, that generate a substantial economic burden for the healthcare system. The vast majority of the associated health resource utilization is derived from a requirement for prolonged hospital stays, numerous outpatient visits and a substantial number of procedures (26). In one study from Spain, the mean cost of a single SRE in patients with MBD was between $2,684 to $8,923 (26). In another similar study out of Spain, the cost of hospital admissions increased as the disease progressed into MBD and subsequent SREs. The average cost of the first admission for those with breast, prostate or lung cancer was $2,775, whereas the average cost of the first admission with MBD increased to $4,112. Finally, the cost of the first admission with a SRE was $4,382 (27).
As cancer progresses and becomes more advanced, the cost of treatment increases. The individual and societal economic burden is higher in patients who develop MBD compared to those who have more localized forms of cancer, and this burden further increases in those who subsequently develop a SRE. Multiple authors have looked into the cost of SREs in individual cancers.
The cost of treating lung cancer patients with MBD is higher compared to patients with MBD of prostate or breast cancer, partly because prostate and breast are often treated with hormone therapy whereas lung cancer treatments include oral targeted therapy and immunotherapy. Furthermore, these patients generally present with significantly advanced disease and have a dismal prognosis. The cost of treatment of SREs in patients with MBD of lung primary was approximately $12,000 in a 2004 study (28). Although this figure may seem relatively small, one must keep in mind that these patients have a shorter mean survival after a SRE. In another study, the monthly cost of treatment for lung cancer patients was $214 for those with asymptomatic bone metastases, $421 for patients with symptomatic bone metastases and $5,258 for patients who endured a SRE (29). In a nationwide study out of Denmark, SREs in lung cancer patients were associated with an increased frequency of hospital contacts and a greater number of hospital days in the SRE diagnostic period and post-SRE period compared to the pre-SRE period (30). SREs are also common among patients with MBD from prostate cancer, and they are significantly more costly for patients who experience multiple SREs. In a retrospective claims analysis, the mean cost per patient (with MBD of prostate cancer) in the year after the initial diagnosis of a SRE was $12,469 (31). Among Medicare beneficiaries, the average healthcare utilization cost of patients with SREs is $29,696 higher than that of prostate cancer patients without SREs (32). In another study among men with prostate cancer and bone metastases, those who experienced a SRE used substantially more health care resources compared to those who remained SRE-free (including ED visits, rate of hospitalizations, rates of ambulatory visits, imaging, surgical procedures, use of bone-sparing agents, and chemotherapy and the prolonged rehabilitation courses). They concluded that the mean per-patient cost attributable to experiencing a SRE was estimated to be $21,191 (19). Similarly, in a study of patients with advanced breast cancer, the authors reported average costs per SRE (fees charged) over 60 months (mean follow-up 13.8 months) among a limited subset of patients with SREs matched to patients without SREs and found that total medical care costs were $48,173 greater in patients with SREs compared to patients without SREs (33). The importance of SREs, from a health economic perspective, in prostate and breast cancers cannot be overstated, given the prevalence of these diseases.
Regardless of the primary tumor, the treatment of patients who develop MBD and SREs remains a significant challenge and burden on any healthcare system. The utilization of health care resources can however vary according to the specific type of SRE. The vast majority of the associated cost is driven by the need for (often lengthy) inpatient stays, outpatient visits, as well as a substantial number of procedures. Of these resources, inpatient stays generally contribute the most to the cost of each SRE type (26).
Bone pain
Skeletal pain secondary to MBD is the most common SRE. Radiation therapy to the affected regions is typically the first line treatment employed, and results in decreased pain and improved function in a majority of patients. Compared with other SREs, radiation therapy to bone is associated with relatively lower average management costs ($2,675) (26). This is mainly due to the fact that it can generally be administered in an outpatient setting. Nonetheless, patients undergoing radiation therapy require more hospitalizations compared to patients with no SREs (34). Among the SREs, radiation therapy for painful skeletal metastases contributes the most to outpatient visits, and the least to inpatient stays (mean of 12 days) (35,36).
Pathologic fractures
The management cost of pathologic fractures without surgery is among the more expensive SREs (26). In a study examining the payer costs for SRE-related hospitalizations among patients with bone metastases, the mean health plan payment per hospital admission reported was $24,224 for pathologic fracture care without surgery (33). These patients require increased number and duration of inpatient stays (mean of 20 days), increased emergency department visits and increased outpatient visits (26,34-36). This particular issue -aside from others analyzed further- emphasizes consideration of the potential benefits that surgical management of SREs has for most patients, especially those presenting with pathological fractures.
Spinal cord compression
Due to the complicated nature of its treatment, spinal cord compression is the SER associated with the highest management costs (26). The mean payer cost per hospital admission for a spinal cord compression event is roughly $54,444 (33). Among the different types of SREs, patients with spinal cord compression require the longest length of inpatient stay (mean of 30 days) and the highest number of hospitalizations (26,35). This SRE type is also associated with the highest number of procedures (35). As with other SRE types, patients with spinal cord compression require significantly higher emergency department and outpatient visits (34,36).
Surgery
Similar to spinal cord compression, surgery primarily on long bones has high management costs, with the mean payer cost per hospital admission being approximately $35,284 (33). Although spinal cord compression and surgery on long bones are both associated with a higher requirement for inpatient stays, the average length of inpatient stay per event is shorter with surgery to appendicular sites (mean of 15 days), and thus the total cost of management is comparatively lower (26,36). The mean number of inpatient stays, the mean length of inpatient stay, the mean number of outpatient visits and emergency department visits are all increased in cases of surgery to the appendicular skeleton (34-36). Pathologic fractures requiring surgery in MBD not only generate substantial costs, but are also traumatic events to patients and their families, both physically and emotionally. They often contribute to adverse events and prophylactic fixation of pathologic lesions prior to the occurrence of a fracture may mitigate their effects. Prophylactic fixation of long bones facilitates earlier ambulation, decreases pain, and has less cost to the hospital, as compared with surgical treatment of pathologic fractures (37). Drivers of increased cost in patients with pathologic fractures treated surgically compared with patients with prophylactic treatment include longer mean hospital length of stays, and more complex postoperative rehabilitation (37,38). In one study, prophylactic fixation decreased mean costs per event by roughly $3,500 when compared to surgically treated pathologic fractures (38). Similarly, in another study, when taking into consideration both direct and indirect costs, prophylactic fixation of pathologic lesions saves approximately $21,000, compared to the surgical treatment of pathologic fractures (37). In evaluating the cost-effectiveness of hip reconstruction in patients with skeletal metastases to the hip, there is a 22- fold reduction in costs among patients who undergo surgery compared to patients who are managed conservatively ($473,279 vs. $21,641) (39). Thus, prophylactic stabilization of metastatic lesions, when appropriate, can be of immense clinical and economic value.
Anti-resorptive therapy: bisphosphonates & denosumab
In MBD, systemic treatments such as radiopharmaceuticals and bisphosphonates are often used to shrink or slow the growth of bone metastases and thus prevent SREs. These treatment options specifically target skeletal metastatic sites. Common bisphosphonates include pamidronate, clodronate and zoledronic acid (ZA). Another systemic treatment includes denosumab: a newer and more expensive human monoclonal antibody, that specifically binds to and blocks activity of the receptor activator of nuclear factor (NF)-kB ligand (RANKL), which mediates the formation, function, and survival of osteoclasts.
While these treatments may reduce the occurrence of SREs, they can potentially come at an additional cost depending on the price of acquisition. In a recent systematic review, the economic valuations and cost-effectiveness of these treatments were reviewed. The authors found that bisphosphonates are associated with an additional cost upwards of $12,300 per SER avoided, and that denosumab is substantially more costly than any of the bisphosphonates. They concluded that, depending upon the acquisition cost, bisphosphonate treatment may be a cost-effective option for the management of bone metastases. However, due to the substantial additional costs of denosumab, its treatment currently does not represent value for money (40).
Societal & patient impact
Many cancer survivors experience substantial financial hardship. The complexity of measuring the financial hardship of cancer has led to substantial heterogeneity in methods and measures. The main domains to analyze are: (I) productivity loss, (II) out of pocket medical care costs, and (III) depletion of assets as a result of the first two domains, leading to an increased risk for medical debt, bankruptcy, and increased stress, anxiety, and worry about finances. In a 2017 review of financial hardships reported by cancer survivors, the prevalence of financial hardship varied by the measure used and population studied. Mean annual productivity loss ranged from $380 to $8,236, 12% to 62% of survivors reported being in debt because of their treatment, 48% of survivors reported experiencing some form of financial distress, and 4% to 45% of survivors did not adhere to recommended prescription medication because of cost (41). As medical costs are increasingly shifted to patients through higher health insurance premiums, deductibles, and greater cost sharing, the ongoing surveillance of the multiple domains of financial hardship will be critical as cancer patients navigate treatment and survivorship. There is a growing need for consistent use of definitions, terms, and measures to better inform development of interventions to lessen future financial hardships of a costly branch of medical care. The costliest time for oncologic care is when the disease is in advanced stage, and palliative care is imminent. Medicare spends 1/3 of the cost of treating cancer in the final year and 78% of that spending occurs in the final month of life (42). When households that had recently experienced a cancer death were surveyed, the results showed that approximately a quarter of respondents reported that the cost of care was a major financial burden, and a third used all or most of their savings to cover costs of care (43).
Caregiver burden
Working-age caregivers face the challenge of meeting work demands while pursuing professional goals and caring for their loved ones. Demands of employment cannot be ignored because employment is essential for family income and/or employment-based health insurance coverage. Estimates of the impact on caregivers’ employment vary considerably by whether the patient is an adult or child, type of cancer the patient has, the stage of diagnosis, and long-term prognosis for the patient. Regardless of these factors, nearly all caregivers lose some time from work while caring for a cancer patient (44).
Potential impacts on a caregivers employment: (I) loss of employment; (II) loss of employer-based health insurance and other benefits; (III) loss of income; retirement savings, and employer contributions; (IV) time away from work (paid and unpaid), (V) reduced work productivity; (VI) working extra hours to compensate for patient’s inability to work and/or loss of income and health insurance or other benefits; (VII) accepting lower paying jobs to accommodate schedule. The threat of employment loss, potential loss of insurance, and exorbitant out-of-pocket costs leave cancer patients and their caregivers vulnerable. Legal protections and insurance options available to cancer patients sometimes extend to caregivers. Although States differ in their provision of protections for caregivers against job loss, the Americans with Disabilities Act (ADA) and the Family Medical Leave Act (FMLA) are the two main federal policies that offer work-place protections to caregivers (44). In a longitudinal study from Canada of 89 breast cancer patients and their caregivers, it was demonstrated that caregivers experienced substantial psychological morbidity (anxiety and depression) at the onset of the patient’s palliative illness, and a substantial increase in caregiver burden and depression when the patient reached a terminal stage of the illness. Caregiver burden was the most important predictor of both caregiver anxiety and depression. In addition to psychological morbidity, caregivers bore both economic and occupational burdens (45). In the absence of sweeping policies that offer sick leave and affordable care, the oncology care team and researchers need to work together to develop interventions, or modify existing interventions, to include a financial component (44,45).
Psychological impact of cancer
Cancer does not only impact a patient’s body and finances; it has a significant effect on their emotional and mental state. Information for cancer patients is vast and includes multiple media outlets. Websites and pamphlets speak about the psychological and emotional impacts of cancer. With the prevalence of these psychological issues affecting cancer patients it is surprising to see limited scientific studies analyzing the assessment and treatment for these secondary complications of bone disease. SRE’s cause significant morbidity and dramatically decrease a patient’s quality of life. A study of breast cancer patients in 2013 analyzed their concerns in personal essays cancer survivors wrote. Their comments and concerns were able to fit into one or more of three main themes. First, treatment may result in several quality-of-life concerns, including physical symptom burden, emotional distress, body image disturbance, and disrupted daily activities. Second, social constraints on disclosure of cancer-related concerns may exacerbate a patients’ distress. Third, many patients experience a heightened awareness of life’s brevity and search for meaning in their cancer experience (38). Most cancer centers provide a multitude of educational information for patients, including multiple psychological and social issues that cancer patients may experience. These include grief, depression, body image concerns, fear of recurrence, spiritual guidance, survival guilt, workplace concerns, anxiety, and relationships. When treatment can include surgery, radiation therapies and medical therapies most, if not all of these emotions, come into play. Many of these feelings arise from impaired mobility, loss of functional independence, and diminished health-related quality of life (15). A study out of the European Society for Medical Oncology in 2004 tried to understand the undiagnosed psychological disorders in cancer patients, and concluded that most patients’ psychological issues go unrecognized, and therefore are unaddressed (46). As advances in treatment for MBD and SRE’s improves, it is key that related psychologic disorders are recognized, understood, and included in the overall treatment of patients with metastatic bone disease. Physical and psychological rehabilitation activities should be practiced periodically, and should be led by professional staff. Long-term educational resources and care should also be provided. In a study involving 80 cancer survivors, heart rate variability was used to efficiently monitor the status of the mind-body balance and it was a more suitable index than questionnaires for physical and psychological function evaluation in cancer survivors (47).
Quality of life measures for patients with MBD
A few centers have answered the call to better evaluate QOL data when looking into treatment decisions in patients with metastatic bone disease. Loss of mobility, functional independence, and health-related quality of life are areas that all future research should take into consideration when evaluating outcomes. The lack of focus on patient-reported outcomes for patients with metastatic bone disease is manifested in the lack of standardized methods to assess health-related quality of life for these patients. Consequently, there is limited data on how to improve health-related quality of life following SRE (15). A recent study conducted at Massachusetts General Hospital, analyzed prospective data from patients with bone metastases derived from solid tumors, myeloma, or lymphoma, who presented to their clinic between 2011–2015. The data consisted of the patients` self-reported outcomes. As part of a quality improvement program, patients completed the following questionnaires before visiting the surgeon at their orthopaedic oncology service since November 2011: the EuroQOL 5 Dimension Questionnaire (EQ-5DTM), Patient- Reported Outcomes Measurement Information System (PROMIS1) Pain Interference, PROMIS1 Anxiety, and PROMIS1 Depression. The authors found that having a pathologic fracture was independently associated with worse QOL, increased anxiety, and more depression. In addition, younger age, current smoking status, and being unemployed were independently associated with worse QOL. Current smoking status was independently associated with more pain interference. A primary tumor type with poor prognosis was associated with more anxiety, and being single was associated with more depression. Patients with metastatic bone disease reported worse QOL, more pain interference, and more anxiety compared with general population values. Physicians can use these factors associated with poorer scores for QOL—pain interference, anxiety, and depression—to anticipate which patients might need additional psychosocial support during treatment for metastatic bone disease. Their study results suggest that impending pathologic fractures should be treated promptly to prevent further deterioration in QOL, anxiety, and depression. Every type of cancer that metastasizes to the bone has a different disease course, treatment, and prognosis; therefore, it would merit further study to reproduce this study in cancer-specific groups, in order to identify more accurate factors accounting for variation in patient-reported outcomes (48). In a multi-center, prospective study from three orthopaedic oncology centers in Quebec, Canada, conducted between 2008 and 2016, the authors found improvement in pain and functional outcome after surgery for patients with long bone metastases. Interestingly, quality of life did not improve (49).
Conclusions
As the prevalence of cancer is increasing, there is an associated rise in the incidence of MBD. Skeletal metastases occur in many malignancies and can subsequently result in SREs. These events fuel the increasing costs of cancer care and strain the economy and healthcare system. From a patient perspective, they are a substantial detriment to personal finances, mental and physical well-being and quality of life. From a caregiver point of view, there are huge financial and psychological implications. The care of patients with MBD can become complicated and challenging. Orthopedic opinions are often sought far too late and earlier referral may offer the opportunity for less complications. The myriad of impacts MBD has on patients, caregivers and society must be taken into consideration by the entire multi-disciplinary team caring for those with advanced cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rui Yang) for the series “Bone Metastasis” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-97/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-20-97/coif). The series “Bone Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1029-36. [Crossref] [PubMed]
- U.S. Census Bureau 2017 National Population Projections Tables: Main Series 2020. Available online: https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html
- American Cancer Society. Cancer Facts & Figures 2020. Am Cancer Soc 2020;1-76. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html
- American Cancer Society. Cancer Treatment & Survivorship Facts Figures 2019-2021. Am Cancer Soc 2019;1-48. Available online: https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html
- Yu HHM, Tsai YY, Hoffe SE. Overview of diagnosis and management of metastatic disease to bone. Cancer Control 2012;19:84-91. [Crossref] [PubMed]
- Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol 2012;4:87-93. [PubMed]
- AAOS. Metastatic Bone Disease 2011. Available online: https://orthoinfo.aaos.org/en/diseases--conditions/metastatic-bone-disease/
- Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer 2007;109:2334-42. [Crossref] [PubMed]
- Hernandez RK, Adhia A, Wade SW, et al. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin Epidemiol 2015;7:335-45. [PubMed]
- Yong M, Jensen AØ, Jacobsen JB, et al. Survival in breast cancer patients with bone metastases and skeletal-related events: A population-based cohort study in Denmark (1999-2007). Breast Cancer Res Treat 2011;129:495-503. [Crossref] [PubMed]
- Liede A, Jerzak KJ, Hernandez RK, et al. The incidence of bone metastasis after early-stage breast cancer in Canada. Breast Cancer Res Treat 2016;156:587-95. [Crossref] [PubMed]
- Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: A review. Crit Rev Oncol Hematol 2005;56:365-78. [Crossref] [PubMed]
- Zickel RE, Mouradian WH. Intramedullary fixation of pathologic fractures and lesions of the subtrochanteric region of the femur. J Bone Joint Surg Am 1976;58:1061-6. [Crossref] [PubMed]
- Damron TA, Mann KA. Fracture risk assessment and clinical decision making for patients with metastatic bone disease. J Orthop Res 2020;38:1175-90. [Crossref] [PubMed]
- Costa L, Badia X, Chow E, et al. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer 2008;16:879-89. [Crossref] [PubMed]
- Ashford RU, Randall RL. Bone metastases: Epidemiology and societal effect. Metastatic Bone Dis An Integr Approach to Patient Care 2015;3-11. Available online: https://ucdavis.pure.elsevier.com/en/publications/bone-metastases-epidemiology-and-societal-effect
- Oster G, Lamerato L, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support Care Cancer 2013;21:3279-86. [Crossref] [PubMed]
- Baek YH, Jeon HL, Oh IS, et al. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: A 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol 2019;61:104-10. [Crossref] [PubMed]
- McDougall JA, Bansal A, Goulart BHL, et al. The Clinical and Economic Impacts of Skeletal‐Related Events Among Medicare Enrollees With Prostate Cancer Metastatic to Bone. Oncologist 2016;21:320-6. [Crossref] [PubMed]
- Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007;110:1860-7. [Crossref] [PubMed]
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis 2011;14:177-83. [Crossref] [PubMed]
- Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol 2013;22:132-8. [Crossref] [PubMed]
- Soni A. Trends in Use and Expenditures for Cancer Treatment among Adults 18 and Older, U.S. Civilian Noninstitutionalized Population, 2001 and 2011. Statistical Brief [Medical Expenditure Panel Survey (US)]. Rockville (MD): Agency for Healthcare Research and Quality (US), 2001.
- Mariotto AB, Robin Yabroff K, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117-28. [Crossref] [PubMed]
- Yabroff KR, Lund J, Kepka D, et al. Economic Burden of Cancer in the US: Estimates, projections and future research. Cancer Epidemiol Biomarkers Prev 2011;20:2006-14. [Crossref] [PubMed]
- Durán I, Garzón C, Sánchez A, et al. Cost analysis of skeletal-related events in Spanish patients with bone metastases from solid tumours. Clin Transl Oncol 2014;16:322-9. [Crossref] [PubMed]
- Pockett RD, Castellano D, Mcewan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755-60. [Crossref] [PubMed]
- Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology 2004;67:390-6. [Crossref] [PubMed]
- Decroisette C, Monnet I, Berard H, et al. Epidemiology and treatment costs of bone metastases from lung cancer: A French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol 2011;6:576-82. [Crossref] [PubMed]
- Skov Dalgaard K, Gammelager H, Sværke C, et al. Hospital use among patients with lung cancer complicated by bone metastases and skeletal-related events: A population-based cohort study in Denmark. Clin Epidemiol 2015;7:363-8. [Crossref] [PubMed]
- Lage MJ, Barber BL, Harrison DJ, et al. The Cost of Treating Skeletal-Related Events in Patients with Prostate Cancer. Am J Manag Care 2008;14:317-22. [PubMed]
- Jayasekera J, Onukwugha E, Bikov K, et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics 2014;32:173-91. [Crossref] [PubMed]
- Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 2010;16:693-702. [Crossref] [PubMed]
- Body JJ, Pereira J, Sleeboom H, et al. Health resource utilization associated with skeletal-related events: results from a retrospective European study. Eur J Health Econ 2016;17:711-21. [Crossref] [PubMed]
- Duran I, Fink MG, Bahl A, et al. Health resource utilisation associated with skeletal-related events in patients with bone metastases secondary to solid tumours: regional comparisons in an observational study. Eur J Cancer Care (Engl) 2017; [Crossref] [PubMed]
- Hoefeler H, Duran I, Hechmati G, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: Results from a multinational retrospective - Prospective observational study - A cohort from 4 European countries. J Bone Oncol 2014;3:40-8. [Crossref] [PubMed]
- Blank AT, Lerman DM, Patel NM, et al. Is prophylactic intervention more cost-effective than the treatment of pathologic fractures in metastatic bone disease? Clin Orthop Relat Res 2016;474:1563-70. [Crossref] [PubMed]
- Mosher ZA, Patel H, Ewing MA, et al. Early Clinical and Economic Outcomes of Prophylactic and Acute Pathologic Fracture Treatment. J Oncol Pract 2019;15:e132-40. [Crossref] [PubMed]
- Singh G, Lim CT, Jonathan TJH, et al. Evaluation of the role and cost-effectiveness of end-of-life orthopaedic interventions in cancer patients with skeletal metastases to the hip. J Palliat Care 2013;29:83-90. [Crossref] [PubMed]
- Andronis L, Goranitis I, Bayliss S, et al. Cost-effectiveness of treatments for the management of bone metastases: A systematic literature review. Pharmacoeconomics 2018;36:301-22. [Crossref] [PubMed]
- Altice CK, Banegas MP, Tucker-Seeley RD, et al. Financial hardships experienced by cancer survivors: A systematic review. J Natl Cancer Inst 2016;109:djw205. [Crossref] [PubMed]
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life associations with End-of-life conversations. Arch Intern Med 2009;169:480-8. [Crossref] [PubMed]
- Cagle JG, Carr DC, Hong S, et al. Financial burden among US households affected by cancer at the end of life. Psychooncology 2016;25:919-26. [Crossref] [PubMed]
- Bradley CJ. Economic Burden Associated with Cancer Caregiving. Semin Oncol Nurs 2019;35:333-6. [Crossref] [PubMed]
- Grunfeld E, Coyle D, Whelan T, et al. Family Caregiver Burden: Results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ 2004;170:1795-801. [Crossref] [PubMed]
- Keller M, Sommerfeldt S, Fischer C, et al. Recognition of distress and psychiatric morbidity in cancer patients: A multi-method approach. Ann Oncol 2004;15:1243-9. [Crossref] [PubMed]
- Lee YH, Lai GM, Lee DC, et al. Promoting physical and psychological rehabilitation activities and evaluating potential links among cancer-related fatigue, fear of recurrence, quality of life, and physiological indicators in cancer survivors. Integr Cancer Ther 2018;17:1183-94. [Crossref] [PubMed]
- van der Vliet QMJ, Paulino Pereira NR, Janssen SJ, et al. What Factors are Associated With Quality Of Life, Pain Interference, Anxiety, and Depression in Patients With Metastatic Bone Disease? Clin Orthop Relat Res 2017;475:498-507. [Crossref] [PubMed]
- Nooh A, Goulding K, Isler MH, et al. Early improvement in pain and functional outcome but not quality of life after surgery for metastatic long bone disease. Clin Orthop Relat Res 2018;476:535-45. [Crossref] [PubMed]
Cite this article as: DiCaprio MR, Murtaza H, Palmer B, Evangelist M. Narrative review of the epidemiology, economic burden, and societal impact of metastatic bone disease. Ann Joint 2022;7:28.


