
Can the Katagiri scoring system predict prognosis for surgically-managed patients with metastatic bone disease?
Introduction
Bone is a frequent site of metastasis that primarily arises from breast, prostate, thyroid, kidney, and lung (1). Metastatic bone disease is a major cause of morbidity and can result in pain, recumbency that can lead to ulceration and muscle atrophy, pathologic fractures, and spinal cord compression (2). Treatment often includes pain management in tandem with the use of anti-resorptive agents, radiation therapy, hormone therapy, surgery, or a combination thereof. Surgical treatment for metastatic disease is generally palliative rather than curative. The goals of surgical management are to reduce pain, achieve structural stability, restore function, and improve quality of life. Life expectancy is frequently considered when weighing the benefits of surgical intervention against its inherent risk and possible complications such as post-operative pain, rehabilitation requirements, hospitalization time, and emotional burden. For this reason, accurate prognostication is essential. For example, overestimation of life-expectancy may result in the patient expiring prior to fully recovering, which can take months in the case of more complex procedures, such as acetabular reconstruction (3). In such instances, regaining mobility and independence might be invaluable in controlling pain, resuming relationships and returning to normal routine activities all of which contribute to quality of life.
Current prognostic systems for surgical patients with osseous metastases include Tokuhashi’s system and Tomita’s system, both of which are limited in that they are primarily designed for patients with spinal metastases (4,5). Prognostic systems for surgical patients with skeletal metastases include the Bauer system and PATHFx. The Bauer scoring system is limited in that it recognizes a small array of primary cancer types and does not take into account laboratory values (6,7). PATHFx is an accurate prognostic tool but it was only validated in surgical treated patients but not in medically treated ones.
The Katagiri scoring system is more comprehensive in that it is applicable to patients with spinal metastases, skeletal metastases, or both. However, to date, it has only been tested in a Japanese population. Moreover, in their report, Katagiri et al. only include a minority of patients that were managed with surgery, making it difficult to draw meaningful conclusions about surgically-managed patients. The purpose of this study was to validate the Katagiri scoring system using a cohort of surgically-managed patients within a diverse tertiary academic medical setting. We hypothesized that the Katagiri scoring system will serve as a reliable predictor of survival for patients with metastatic bone disease who undergo surgical management. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aoj-20-111).
Methods
Eligibility
An IRB-approved retrospective review was performed at Montefiore Medical Center, which is a large tertiary-care academic medical health system serving a diverse patient population. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional board of Albert Einstein College of Medicine and informed consent was taken from all the patients. Patients were identified using Clinical Looking Glass® (CLG) and cross referenced with the institutional Cancer Registry. CLG is an institutional proprietary software application developed for the purpose of assessing and analyzing heath care and outcomes using both clinical and administrative datasets (8). The Cancer Registry compiles and records numerous patient and cancer-related variables including living status. It draws information using numerous data sources including the hospital’s electronic medical record, Social Security Death Index, the New York State Department of Health Death Report, IMPAC’s Deathmatch and Lexis Nexis (Albany, NY, USA). Lexis Nexis, in turn, searches for a patient’s last known living date using publicly available records. Institutional Cancer Registry follow up rate is currently assessed in real-time while prior to 2018, follow up was assessed monthly. Follow-up is in accordance with American College of Surgeons Commission on Cancer Accreditation and is in excess of 95%.
A search for eligible patients was conducted over a 13-year period, from 2005 to 2017. Inclusion criteria included patients with a known diagnosis of metastatic bone cancer who underwent surgical intervention for management of their osseous disease within the institution and who had a minimum of 24 months follow-up. Patients undergoing surgery outside the health system, patients treated non-operatively, and patients who either did not have adequate follow-up or who were lost to follow-up were excluded. Additionally, patients with insufficient documentation or medical data were excluded.
Prognostic variables and data collection
Medical charts were reviewed to identify the relevant data points required for the Katagiri scoring system (9). These included the primary tumor, the growth rate of the primary cancer, the presence of metastasis, various laboratory data (including C-reactive protein, albumin, lactate dehydrogenase, platelet, calcium, and bilirubin levels), the Eastern Cooperative Oncology Group Performance Status (ECOG PS), a prior history of chemotherapy treatment, and the presence of multiple skeletal metastasis (9). Neurologic deficit (Frankel type) and primary tumor type were not considered given that these variables previously failed to reach statistical significance. Similarly, the presence or absence of a pathologic fracture was not considered given that it has previously not been used in the Katagiri scoring system.
Based on the information obtained from the initial chart review, each patient received a score from 0–10. The score was composed of summed prognostic values, with weighting of each value was determined from the natural logarithm of the hazard ratio, as described in the revision of the original scoring system by Katagiri et al. in 2014 (9). Prognostic values such as primary site and rapid growth were assigned 3 points. Critical laboratory data and moderate growth were each assigned 2 points. Disseminated and ordinary metastasis, abnormal laboratory data, poor ECOG PS, previous chemotherapy, and multiple bone metastases were each assigned 1 point. Summed scores ranged from 0–10 and patients were stratified into either a low-risk group (score 1–3), and intermediate-risk group (score 4–6), or a high-risk group (score 7–10).
Statistical analyses
All statistical analyses were performed using SPSS. Chi-square test was performed for 6-, 12- and 24-month predicted survival periods. Yates correction was used for groups consisting of less than five patients. Level of significance was set at 5% (P<0.05). Kaplan-Meier survival curves were generated for low-, intermediate-, and high-risk groups as defined by the Katagiri score.
Results
One hundred eighty-three surgical patients were included in the analysis. There were 77 males and 106 females with a median age of 70. The percentage of patients over the age of 65 was 67.8%. Lung carcinoma was the most common primary lesion. Other common primary sites include breast cancer, prostate cancer, multiple myeloma, and renal cell carcinoma. Table 1 summarizes the types of primary tumors. The most common surgeries performed in this cohort consist of open reduction internal fixation, hemiarthroplasty, intramedullary nail fixation, excision with endoprosthetic reconstruction, and total hip arthroplasty. The most common locations in which these surgeries were performed include the femur, hip, and humerus.
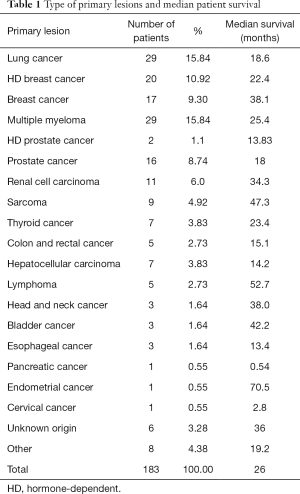
Full table
Among these patients, 27% had an ECOG score between 0 and 2, while 73% of patients had an ECOG score between 3 and 4. The percentage of patients that had normal, abnormal, and critical laboratory data were 64.0%, 20.7%, 15.3% respectively. The percentage of patients with slow growth, moderate growth, and rapid growth were 48.6%, 19.1%, 30.1% respectively. The percentage of patients who did not have visceral or cerebral metastases were 57.4%, while 26.8%, 4.9%, and 10.9% had nodular, visceral or cerebral, and disseminated metastasis respectively. The percentage of patients previously treated with chemotherapy was 44.2%, while the percentage of patients with multiple skeletal metastases was 58.5%. These prognostic factors are summarized in Table 2. After stratifying patients into low-, intermediate-, and high-risk groups based on their Katagiri scores, the survival rates of each group were determined at 6-, 12-, and 24-month (Table 3). The survival rates of our low-risk and high-risk surgical group were compared on a Kaplan-Meier survival curve, demonstrating that the high-risk group has worsening survival rates relative to the low-risk group (Figure 1).
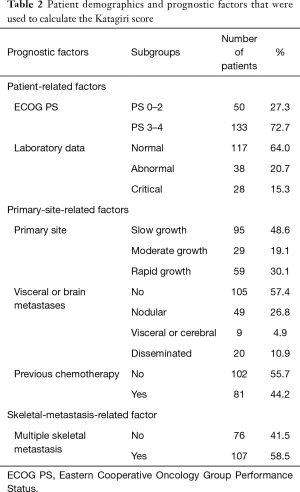
Full table
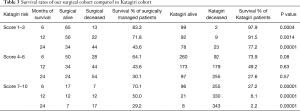
Full table
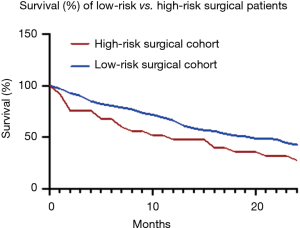
Survival rates of our surgical cohort were compared with the original Katagiri cohort at 6-, 12-, and 24-month. Survival for low-risk surgical patients at 6-, 12-, and 24-month was 83.3%, 71.8%, and 43.6% respectively compared with 97.9%, 91.5% and 77.2% for the Katagiri cohort. These discrepancies in survival rates were significant (P<0.001 for all time points; Table 3). Survival for intermediate-risk surgically-treated patients was 64.1%, 43.6%, and 30.1% compared with 73.9%, 49.2%, and 27.6% for the Katagiri cohort at 6, 12, and 24 months respectively (P=0.08, P=0.63, and P=0.57 respectively; Table 3). Survival rates for high-risk surgical patients at 6-, 12-, and 24-month were 70.1%, 50.0%, and 29.2% respectively compared with 27.2%, 6.1%, and 2.2% for the Katagiri cohort (P<0.001 for all time points; Table 3).
Discussion
Approximately 400,000 patients develop metastatic bone disease annually in the United States, of which around half experience a skeletally-related event (10). These events tend to lead to loss of functional independence and a marked reduction in quality of life, and intervention is often aimed at restoring both. However, surgical intervention carries inherent risk and should be carefully considered, taking into account numerous factors, including life expectancy and overall prognosis. Accurate prognostication is essential in both extending surgical options to patients that stand to benefit and cautioning against surgical intervention in cases where it is unlikely to yield improvement.
The present study has a number of recognized limitations. There are inherent differences between our cohort, which is based on the United States population and the Katagiri cohort, which is derived from the Japanese population. Additionally, the study was retrospective and patients were not randomly assigned to treatment, which introduces selection bias. Decisions on which treatment patients received was made by the treating surgical team and was not based on a standardized protocol. There were a small number of histology-specific malignancies, which introduces a lack of representation of all the various primary tumor types. For instance, only one patient with cervical cancer was included in our study. Our study is also limited in that it is based upon a smaller cohort than that used by Katagiri et al. and a larger cohort would likely be helpful in validating our findings. Medical oncology is also a rapidly evolving field, and although our cohort overlaps temporally with the Katagiri cohort, advances in chemotherapy are constantly changing the prognoses of these patients.
There are a number of scoring systems that predate the Katagiri system. The Tokuhashi scoring system, first introduced in 1989, aimed to define prognosis for patients with spinal metastatic tumor (5). The Tomita scoring system, first introduced in 2001, also aimed to estimate prognosis for metastatic spinal tumors (4). The Tokuhashi and the Tomita scoring systems are often used simultaneously for prognostication when contemplating surgical intervention for patients with skeletal metastasis but is not applicable to patients with skeletal metastasis. Conversely, both the Bauer score and PATHFx can be applied to patients with skeletal metastases. The Bauer scoring system, first introduced in 1995, aimed to define prognosis for patients with either spinal or skeletal metastatic tumors. It was formulated using a cohort comprised of 153 patients with limb bone metastases and 88 patients with spinal metastases (6). Although the Bauer scoring system addresses extremity metastases, it exhibits certain limitations. For instance, it only recognizes four primary cancer types within its calculation, and it does not take into account patient laboratory values, which are useful indicators of prognosis for some malignancies (7). Recognizing this limitation, Ghori et al. subsequently reported survival rate in a cohort of surgically-treated patients using a modified version of the Bauer score that took into account serum albumin, which proved to be a better predictor compared to the original Bauer scoring system (11). Katagiri et al. recognized the importance of laboratory data as a prognostic indicator, which is reflected in their updated scoring system (9). The online tool PATHFx is modeled after Bayesian Belief Network modeling, which was first introduced by Forsberg et al. in 2017 (12). This tool aimed to provide objective survival estimates for patients with skeletal metastases. PATHFx is considered to be a clinically useful tool since it provides accurate prognosis at 1-, 3-, 6- and 12-month and was found to preserve its accuracy when tested in different patient populations (12-15). Although useful, it is not an easily accessible resource and the algorithm from which it was derived has not been disclosed, making it difficult to use and evaluate. Unlike PATHFx, the Katagiri scoring system is an accessible tool that is easy to use and which is based upon readily discernable values or variables.
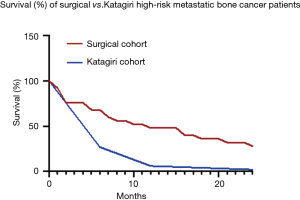
Katagiri et al. found that their cohort of high-risk patients have a survival rate of 27% at 6 months and only 6% at 1 year. Based on these findings, the authors concluded that these patients should not receive invasive treatment. However, in the current surgical cohort, there was a survival rate of 71% at 6 months and 50% at 1 year. The Kaplan-Meier survival curve shows the significant difference in survival rates in the high-risk groups in the surgical cohort and the Katagiri cohort (Figure 2). In the first initial months, survival outcomes are poor for both groups. However, after 2 months the survival outcomes diverge such that our high-risk surgical group have a steady survival outcome compared to the high-risk Katagiri group where the outcome continually worsens. This change in our high-risk surgical group can be explained by post-surgical complications in the initial months leads to worse survival outcomes. Patients that were able to endure those months were able to see the benefits of their surgery in the following months. This suggests that these patients do in fact benefit from surgical intervention and surgeons should strongly consider surgical intervention as a means of improving patient survival outcomes.
Conversely, Katagiri et al. found their cohort of low-risk patients to have a survival rate of 98% at 6 months and 91% at 1 year. Based on these findings, they concluded that these patients should preferably receive long-course radiotherapy and excision followed with reconstruction if surgery is required (9). However, the current surgical cohort demonstrated a survival rate of 83% at 6 months and 72% at 1 year. The Kaplan-Meier survival curve shows a significant difference in survival rates in the low-risk surgical cohort and the Katagiri cohort (Figure 3). The surgical cohort exhibited progressively worsening survival rates that stretched over the ensuing 24 months, a finding which is unlikely attributed to post-surgical complications, which are generally more proximate to the surgery. An alternate explanation could be that these patients required a peri-operative interruption in their systemic chemotherapy treatment to allow for surgery and subsequent wound healing. Admittedly, this is a speculative but reasonable possibility.
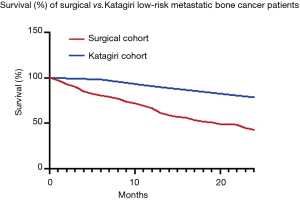
Additionally, while an impending or a complete pathologic fracture is a fairly straightforward indication for surgery, it is not a factor that is used in the Katagiri score. Since pathologic fracture is not accounted for in the score, this could explain the decreased survival rates in our low-risk surgical group compared to the Katagiri et al. cohort. Pathological fracture has been shown to negative prognostic factor for patient outcomes in other studies, but did not influence survival in the Katagiri cohort, hence its exclusion from the scoring system (6,9). In our low-risk surgical group, pathological fracture was present in 83%, compared to 55% overall for the Katagiri cohort. Taken together, these findings suggest that low-risk patients without pathological fracture might benefit from a continued focus on systemic treatment such as chemotherapy if surgery can be safely avoided.
There was no significant difference in the survival rates of the intermediate-risk groups, which suggests that Katagiri score predicts well for surgical patients with intermediate-risk. Therefore, the intervention given in this category should be evaluated based on patient-specific characteristics and patient goals.
Based on our results, the Katagiri scoring system seems to overestimate the survival for lower risk surgical patients while underestimating survival for higher risk patients. Determining prognosis is notoriously challenging, especially in oncology, and cannot be reduced to a scoring system. However, our findings can provide clinicians with additional information when considering the clinical relevance of the Katagiri score for their individual patient.
In conclusion, the Katagiri scoring system predicts survival well for surgically-managed patients within the intermediate-risk group. However, it underestimates the survival of surgically managed patients in the high- and low-risk groups. Future directions include assessing the Katagiri scoring system in a larger surgical cohort and evaluating a modified Katagiri scoring system that takes into account pathologic fracture.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Bone Metastasis”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aoj-20-111
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoj-20-111
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-111). The series “Bone Metastasis” was commissioned by the editorial office without any funding or sponsorship. RY served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional board of Albert Einstein College of Medicine and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s. [Crossref] [PubMed]
- Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res 2016;36:2631-7. [PubMed]
- Marco RA, Sheth DS, Boland PJ, et al. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am 2000;82:642-51. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand 1995;66:143-6. [Crossref] [PubMed]
- Cassidy JT, Baker JF, Lenehan B. The role of prognostic scoring systems in assessing surgical candidacy for patients with vertebral metastasis: a narrative review. Global Spine J 2018;8:638-51. [Crossref] [PubMed]
- Bellin E, Fletcher DD, Geberer N, et al. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med 2010;85:1362-8. [Crossref] [PubMed]
- Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359-67. [Crossref] [PubMed]
- Smith H, Navani A, Fishman SM. Radiopharmaceuticals for palliation of painful osseous metastases. Am J Hosp Palliat Care 2004;21:303-13. [Crossref] [PubMed]
- Ghori AK, Leonard DA, Schoenfeld AJ, et al. Modeling 1-year survival after surgery on the metastatic spine. Spine J 2015;15:2345-50. [Crossref] [PubMed]
- Forsberg JA, Wedin R, Boland PJ, et al. Can we estimate short- and intermediate-term survival in patients undergoing surgery for metastatic bone disease? Clin Orthop Relat Res 2017;475:1252-61. [Crossref] [PubMed]
- Meares C, Badran A, Dewar D. Prediction of survival after surgical management of femoral metastatic bone disease - a comparison of prognostic models. J Bone Oncol 2019;15:100225 [Crossref] [PubMed]
- Ogura K, Gokita T, Shinoda Y, et al. Can a multivariate model for survival estimation in skeletal metastases (PATHFx) be externally validated using Japanese patients? Clin Orthop Relat Res 2017;475:2263-70. [Crossref] [PubMed]
- Piccioli A, Spinelli MS, Forsberg JA, et al. How do we estimate survival? External validation of a tool for survival estimation in patients with metastatic bone disease-decision analysis and comparison of three international patient populations. BMC Cancer 2015;15:424. [Crossref] [PubMed]
Cite this article as: Ugur E, Doshi H, Wilson S, Levine NL, Tingling J, Yang RY, Hoang BH, Geller DS, Yang R. Can the Katagiri scoring system predict prognosis for surgically-managed patients with metastatic bone disease? Ann Joint 2021;6:29.


