Massive pseudotumor of unknown etiology in a cemented metal-on-polyethylene total hip arthroplasty: a case report
Introduction
The formation of destructive pseudotumors is a well-documented, albeit rare, complication of total hip arthroplasties (THA) (1). Although most commonly associated with metal-on-metal (MoM) bearings, case reports of pseudotumors have been published on metal-on-polyethylene (MoP) and metal-on-ceramic (MoC) bearings (2-6).
These pseudotumors are non-infectious and non-neoplastic adverse soft tissue reactions with osteolysis surrounding THA implants. They may appear as periprosthetic granulomatous masses or destructive acetabular lesions. Though they are non-neoplastic, these lesions tend to be progressive and, if left untreated, could result in extensive periprosthetic bony destruction. Since pseudotumors exist on a spectrum from soft tissue mass to extensive bony destruction, the treatment can vary significantly, with the need for complex revision implants to address the resultant pelvic deficiency.
We herein present a case of massive pseudotumor formation of unknown etiology with extensive progression and pelvic destruction 35 years following a primary cemented MoP THA that was managed with palliative intralesional debulking and removal of hardware without reconstruction. The following case is presented in accordance with the CARE reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-3/rc).
Case presentation
Ethics statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). A discussion was had with the patient and her family regarding the data shared in the case report and informed consent was obtained.
Clinical presentation
A wheelchair bound 86-year-old woman presented to the emergency room in May of 2018 with acutely worsening pain following a 10-year history of progressive left hip pain, enlarging pelvic mass, and shortening of her left lower extremity. She had a history of a primary left THA in 1983 at an outside hospital. Based on the radiographs and operative findings, the implants used were a cemented Protek monoblock femoral component (Sulzer Orthopaedics, Switzerland) with a cemented polyethylene cup. She reported significant, progressive pain and dysfunction and was unable to ambulate or sit upright at the time of presentation. Her past medical history was significant for a pacemaker and aortic valve replacement secondary to aortic stenosis and rectal cancer treated with radiation therapy to the pelvic region 8 years ago.
Physical examination
On examination, there was a large, well circumscribed, fixed mass in the left pelvic and upper thigh region, which was firm and tender. It extended from the mid-thigh up into the lower abdomen. There was no neurovascular compromise. The patient was unable to ambulate or sit comfortably in a wheelchair due to mass effect. General examination was otherwise unremarkable. Laboratory investigations were significant for a C-reactive protein (CRP) of 5.3 mg/dL (<1 mg/dL), white blood cell count of leukocytes 7,400/mm3 (3,500–12,000/mm3), and a hemoglobin of 7.8 g/dL (12.0–16.0 g/dL).
Imaging
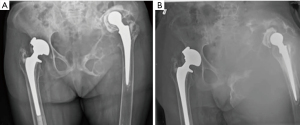
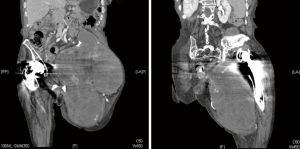
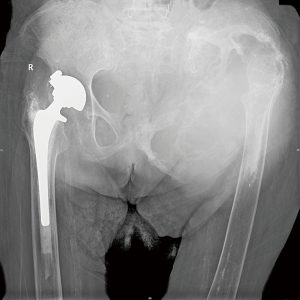
Previous radiographs demonstrated a lytic process involving the left hemipelvis causing superior migration of both the femoral and acetabular implants in situ (Figure 1). A biopsy was performed eight years prior to presentation, which demonstrated scant fibrous tissue with histiocytes and fragments of tissue with features suggestive of a benign vascular lesion. There was no further work up at that time due to patient co-morbidities. Radiographs of the pelvis and femur at presentation demonstrated interval progression of the destructive lytic process of the left hemipelvis (Figure 1). Computed tomography (CT) scan demonstrated a destructive expansile bone lesion of the left hemipelvis measuring 17 cm × 23 cm × 34 cm, extending from the left mid abdomen into the left upper thigh (Figure 2). Although a metal artifact reduction sequence (MARS) magnetic resonance imaging (MRI) is the gold standard for assessing potential pseudotumors, the patient’s pacemaker was a contraindication and MRI was not performed.
Ultrasound-guided core needle biopsy was non-specific and demonstrated benign fibrin- containing tissue consistent with chronic hematoma formation. No evidence of malignant tissue was seen. Additionally, no polyethylene debris, metal particles and histiocytic reaction were noted in the tissue biopsy.
Management
Despite a non-diagnostic biopsy, the clinical history and radiographic findings led us to a presumptive diagnosis of pseudotumor secondary to particle wear. Given the significant bony destruction and the frailty of the patient, it was decided that joint reconstruction was not a viable option. The patient underwent removal of the arthroplasty implants and intralesional pseudotumor debulking as a palliative measure (Figure 3). She was placed in the lateral decubitus position and the joint was entered through the original lateral incision. The prosthesis and surrounding cement was removed first without incident. The acetabular cement mantle was frankly loose within the residual bone but the cup-cement interface was intact and there was significant wear of the polyethylene. The femoral cement mantle was partially fragmented and the stem was removed easily but as the femoral component was monoblock no taper corrosion was possible and there was no evidence of other damage to the stem. A total of 3,500 grams of fragmented hemorrhagic tissue was removed intralesionally from the pseudotumor (Figure 4). Jackson-Pratt (JP) drains were placed and remained in situ until output had reduced to an acceptable level.
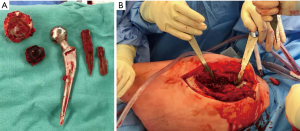

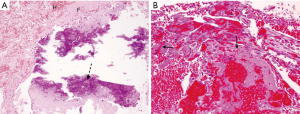
The final pathology was again non-specific and did not support the diagnosis of particle disease. Gross pathology examination demonstrated multiple fragments of friable hemorrhagic tissue measuring 56 cm in aggregate (Figure 4). The tissue has been extensively sampled and 40 sections were submitted for histologic examination. Microscopic sections showed tissue mostly composed of fibrin and blood with multiple foci of calcification and reactive papillary endothelial hyperplasia. These features can be seen in remote hematoma; however, hemosiderin pigment deposits were not detected. There was no evidence of particle debris or macrophages (Figure 5).
At one year follow-up, the patient reported significant pain relief. At last follow-up she was able to ambulate safely with gait aids. Her hemoglobin stabilized post-operatively and ongoing transfusions were not required. Follow-up radiographs showed no further progression of the lytic lesion (Figure 6).
Discussion
The current case presents the diagnostic dilemma of a large benign mass consistent with a pseudotumor on both imaging and intraoperative findings but histologic findings demonstrating chronic hematoma. It highlights the importance of close follow-up and early intervention when periprosthetic osteolysis is detected.
The most concerning differential diagnosis for the patient described in this case report was a radiation-induced sarcoma. The destructive nature of the lesion, the patient’s history of previous colorectal cancer, and subsequent radiation to the region put sarcoma on our differential diagnosis list. Recent registry data has demonstrated that rectal cancer survivors who undergo radiotherapy had a significantly increased risk of developing a sarcoma when compared to survivors who did not undergo radiotherapy (7). However, given the chronicity of the lesion and the lack of malignancy or any neoplastic process confirmed on the initial core biopsy, a diagnosis of radiation-induced sarcoma was lower on the list of possible diagnoses.
Highest on our differential diagnosis was a pseudotumor; defined as a cystic lesion associated with a THA that is not infectious or neoplastic in nature. The term pseudotumor was coined to describe the adverse reaction to metal that a subset of patients have in response to MoM THAs (1). These pseudotumors are well documented and occur in response to high wear metal debris or in patients with metal hypersensitivities (3,8). Pseudotumors in MoM have been described as aseptic lymphocytic vasculitis-associated lesions and demonstrate reproducible findings on histopathology (3). Pseudotumors secondary to metal debris can also occur in non-MoM implants. Mao et al. published a case of pseudotumor formation with pathologic features consistent with metallosis in a MoP and concluded that significant metal wear at the head-neck taper caused metal debris and subsequent inflammation (9).
Polyethylene debris can also be a source of foreign body reactions leading to granulomatous based pseudotumors (4,10-12). There are several cases of pelvic masses and osteolysis associated with THAs secondary to polyethylene debris (13,14). The polyethylene debris is consumed by macrophages and inflammatory cells causing a foreign body-type reaction leading to cyst formation and osteolysis (12,15). Osteolysis secondary to the foreign body-type reaction can occur throughout the joint capsule and may even extend outside of the joint if a defect develops (5,16). The majority of these cases demonstrate giant cells, macrophages, eosinophils, and microscopic polyethylene debris.
There are case reports of chronic expanding hematomas in the setting of hip arthroplasty in the literature. Goddard et al. describe a case of an expanding hematoma after a revision hip arthroplasty for aseptic loosening (17). The few cases that have been published describe inciting events leading to hematoma formation including revision surgery, trauma and the use of anticoagulants (17-19). To our knowledge, our case is the first in the literature to present with findings consistent with both polyethylene wear and chronic hematoma formation.
Our case presents an interesting diagnostic dilemma. The clinical presentation, intraoperative findings, and imaging are certainly consistent with pseudotumor formation and osteolysis secondary to foreign debris. There was significant wear of the polyethylene cup, however the typical histologic findings of polyethylene wear particle-related histiocytic reaction were not seen. Our hypothesis is that the lytic process and pseudotumor was likely initiated by particle debris and subsequent pseudotumor given the significant polyethylene wear. The ensuing bone loss and subsequent instability could have certainly led to hematoma formation and further bony destruction which explains the histologic findings. A combination of polyethylene debris and pseudotumor followed by chronic hematoma formation and further bony destruction is the most likely etiology.
This case highlights the importance of long-term follow-up and early intervention when signs of periprosthetic osteolysis are seen. Radiographic signs of osteolysis were seen dating back 9 years prior to her presentation to hospital. Earlier workup and definitive surgical management would have allowed for more reconstructive surgical options that may have resulted in improved functional outcome for this patient.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-3/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-3/coif). MG declares personal fees from Wright Medical and Amgen, grants from the Canadian Institutes of Health Research, the Canadian Cancer Society and Hamilton Academic Health Sciences, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). A discussion was had with the patient and her family regarding the data shared in the case report and informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davis DL, Morrison JJ. Hip Arthroplasty Pseudotumors: Pathogenesis, Imaging, and Clinical Decision Making. J Clin Imaging Sci 2016;6:17. [Crossref] [PubMed]
- Fehring TK, Odum S, Sproul R, et al. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res 2014;472:517-22. [Crossref] [PubMed]
- Campbell P, Ebramzadeh E, Nelson S, et al. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res 2010;468:2321-7. [Crossref] [PubMed]
- Park YS, Lim SJ, Kim JH, et al. Thigh mass associated with polyethylene wear-induced osteolysis after cementless total hip arthroplasty. Arch Orthop Trauma Surg 2010;130:1097-101. [Crossref] [PubMed]
- Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 1992;74:849-63. [Crossref] [PubMed]
- Hsu AR, Gross CE, Levine BR. Pseudotumor from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2012;41:422-6. [PubMed]
- Berrington de Gonzalez A, Kutsenko A, Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res 2012;2:18. [Crossref] [PubMed]
- Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br 2008;90:847-51. [Crossref] [PubMed]
- Mao X, Tay GH, Godbolt DB, et al. Pseudotumor in a well-fixed metal-on-polyethylene uncemented hip arthroplasty. J Arthroplasty 2012;27:493.e13-7. [Crossref] [PubMed]
- Goto T, Mineta K, Takasago T, et al. Pseudotumor associated with cemented bipolar hemiarthroplasty: an unusual presentation as a granulomatous thigh mass. Skeletal Radiol 2015;44:1541-5. [Crossref] [PubMed]
- Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol 2011;77:43-50. [Crossref] [PubMed]
- Santavirta S, Konttinen YT, Bergroth V, et al. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J Bone Joint Surg Am 1990;72:252-8. [Crossref] [PubMed]
- Wang JW, Lin CC. Pelvic mass caused by polyethylene wear after uncemented total hip arthroplasty. J Arthroplasty 1996;11:626-8. [Crossref] [PubMed]
- Leigh W, O'Grady P, Lawson EM, et al. Pelvic pseudotumor: an unusual presentation of an extra-articular granuloma in a well-fixed total hip arthroplasty. J Arthroplasty 2008;23:934-8. [Crossref] [PubMed]
- Jacobs JJ, Shanbhag A, Glant TT, et al. Wear Debris in Total Joint Replacements. J Am Acad Orthop Surg 1994;2:212-20. [Crossref] [PubMed]
- Hisatome T, Yasunaga Y, Ikuta Y, et al. Hidden intrapelvic granulomatous lesions associated with total hip arthroplasty: a report of two cases. J Bone Joint Surg Am 2003;85:708-10. [Crossref] [PubMed]
- Goddard MS, Vakil JJ, McCarthy EF, et al. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty 2011;26:338.e13-5. [Crossref] [PubMed]
- Ando W, Yamamoto K, Koyama T, et al. Chronic Expanding Hematoma After Metal-on-Metal Total Hip Arthroplasty. Orthopedics 2017;40:e1103-6. [Crossref] [PubMed]
- Uchida K, Negoro K, Kokubo Y, et al. Retroperitoneal hematoma with bone resorption around the acetabular component after total hip arthroplasty: a case report and review of the literature. J Med Case Rep 2012;6:294. [Crossref] [PubMed]
Cite this article as: Gazendam A, Masrouha K, Popovic S, Ghert M, Wilson D. Massive pseudotumor of unknown etiology in a cemented metal-on-polyethylene total hip arthroplasty: a case report. Ann Joint 2022;7:40.