
The discoid lateral meniscus in children: a narrative review of pathology, diagnosis and treatment
Introduction
Discoid lateral meniscus (DLM) is a congenital abnormality of the meniscal shape, characterized by a typical central hypertrophy and a diameter larger than a regular meniscus. This deformity leads to a loss of the typical “C-shape”. DLM was described for the first time in 1887 by Young through cadaveric dissection on lateral meniscus (LM) (1), while in 1930 Watson-Jones discovered the same anomaly also in the medial side (2). The incidence of discoid meniscus is about 0.4–17% for the lateral and 0.1–0.3% for the medial, although, being often asymptomatic, the true prevalence is unknown (3). According to the literature, Asiatic population have a higher rate of DLM (13%) compared to other populations (4-8). Bilateralism is described in 79–95% of cases (8-11).
The purpose of this paper is to provide an update and a general review of this uncommon meniscal pathology. We begin with an overview of anatomic abnormalities of DLM in comparison with a normal meniscus, then discuss the symptoms onset and the current guidelines for clinical management. Finally, we present the current surgical options for painful DLM. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-21-31/rc).
Methods
A literature search performed in PubMed (all publication until October 2021) using the terms “Discoid Lateral Meniscus” and “Children”, yielded 534 titles. Articles were included when they were written in English and when they addressed the etiology, diagnosis, and management of DLM in children or in patient younger than 18 years. References of the included studies were checked for additional studies meeting the inclusion criteria (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | November 17, 2021 |
| Databases and other sources searched | PubMed |
| Search terms used (including MeSH and free text search terms and filters) | Search terms: “Discoid Lateral Meniscus”; “Children” |
| Timeframe | From origin until October 31, 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Inclusion criteria: (I) original articles about DLM; (II) written in English; (III) reporting etiology, diagnosis and management of DLM; (IV) performed in children (<18 years of age at the time of surgery); (V) involving three or more cases; (VI) peer reviewed |
| Exclusion criteria: (I) studies not reporting original research, including review articles, expert opinion or current concepts articles; (II) posters or abstracts at annual meetings or masters’ theses without subsequent peer-reviewed publication of an article; (III) articles not written in English; (IV) studies reporting only adult cases (>18 years); (V) case reports or studies reporting less than 3 cases | |
| Selection process | Two non-blinded authors reviewed the titles and abstracts of each article identified in the literature search. If a study met all the criteria or the abstract did not provide enough information to include or exclude the report, full texts were obtained, reviewed and considered for data extraction. Whenever an agreement about study inclusion could not be resolved by consensus between the two reviewers, a third author decided about the inclusion |
DLM, discoid lateral meniscus.
A total of 76 studies were found with relevant information or data on one or more of the study questions.
Anatomy and etiology
The LM is a crescent-shaped fibrocartilaginous structure, allocated between the lateral femoral condyle and the lateral tibial plateau, that is covered for approximately 75–93% of its entire area, with a slight decrease with age (12). LM is wedge-shaped in cross-section, having a mean coronal width of 9±2 mm, not significantly increasing during growth, a mean peripheral height of 4–5 mm and a mean sagittal width of 18±4 mm, that significantly increase during growth (12). The LM has only loose peripheral attachments to the joint capsule (coronary ligament or popliteomeniscal fascicles), that are interrupted by the popliteal tendon at the popliteal hiatus. Two recent studies have shown that the fibers from the horns of LM have an anatomic relationship with anterior cruciate ligament (ACL), in particular the outer fibers from the anterior horn joined ACL, while the inner fibers from the anterior horn constituted the lateral margin of the ACL attachment. Although fibers from LM seemed to blend with ACL in the macroscopic observation, there is a clear border between ACL and LM in histological study (13,14). The LM has no additional attachments to the corresponding collateral ligaments, that allowing for increased mobility, compared with the medial meniscus. If present, the meniscofemoral ligament (MFL) additionally attaches to the LM. The MFL arises from the posterior horn of the LM and passes to attach to the lateral aspect of the medial femoral condyle. It splits into two bands at the posterior cruciate ligament: anterior MFL (Humphrey) and posterior MFL (Wrisberg). Nonetheless, the presence of the MFLs is inconstant in the general population, with approximately 20% people lacking both ligaments, 50–60% having at least one band (being the posterior band more represented), and about 25–30% people having both.
Histologically, meniscal tissue is composed by 75% of water, 20% type I collagen and 5% other components like elastin and proteoglycans (15). Thanks to its geometry and histological structure, the main role of the LM is to redistribute the contact forces across the tibiofemoral articulation and to augment the knee stability and congruity during motion. Menisci develops by mesenchymal tissue that starts differentiating at the 8th gestation week. It assumes the characteristic crescent shape around the 9th week and keeps maturing until the 14th week, establishing relationship with the other knee structures (16). During the embryological development, menisci are fully vascularized. Starting from 9th month of life, the central zone starts becoming avascularized until the blood-flow is limited to the peripheral third in adulthood. A congenital variant of the normal morphology of the LM is the DLM. Smillie suggested that this variation in structure is due to a failed involution from the embryonal discoid structure to the adult C-shaped profile (4). Other studies showed that the meniscal discoidal shape is not a normal stage of embryonal development of it. Kaplan was the first to support a correlation between the formation of DLM and an abnormal relationship with other articular structures (17). He hypothesized that the high mobility of the meniscus, caused by a deficit of the posterior stabilizers, could lead to a progressive formation of a discoid shape, even in an originally normal meniscus. This theory, however, cannot explain the origin of a medial discoid meniscus, thus currently the most accredited hypothesis is a synergic process between discoid shape and instability. The extracellular matrix (ECM) component in a discoid meniscus is similar to that present in a normal meniscus (collagen fibers, fibrils, and proteoglycans). The presence of organized ECM in meniscal samples collected from multi-organ donors, with homogenous distribution and orientation of collagen fibers with the characteristic periodic organization and diffuse proteoglycans, was already described by histological examination with Safranin O-Fast Green (S-O-FG) staining and confirmed by transmission electron microscopy (TEM) (18) (Figure 1A,1B). On the contrary, the main histological feature of discoid meniscus is a severe disorganization of the collagen fibers loss of structure with bands and degenerated ECM with mostly disorganized collagen fibers and proteoglycan deposition (Figure 1C,1D) and already described by Bisicchia et al. (19).
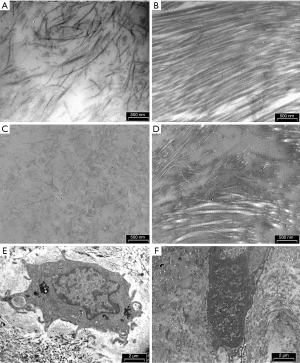
The cells in the DLM sample presented the nuclear chromatin more condensed (Figure 1E) compared to that in the healthy menisci of the multi-organ donors (Figure 1F). Chromatin margination and condensation with a specific pattern, termed by Roach et al. (20) as “chondroptosis” is a sign of a highly-regulated, active process of cell death involved in development, homeostasis and aging. In particular, in pathological cells there are an increase of chondroptosis. In the cytoplasm we observed abundant vacuoles but sparse cytoplasmic organelles, and autophagic vacuoles, presumably due to oxidative stress. As already observed by Battistelli et al. in the menisci of multi-organ donors, the cells embedded in the ECM showed rounded healthy morphology, characteristic of viable and metabolically active cells (18). The nuclei exhibited diffuse chromatin and the cytoplasm contained a high amount of glycogen, mitochondria were round and swollen, and the rough endoplasmic reticulum was well preserved.
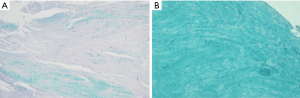
A weak staining intensity for S-O-FG was observed in DLM sample, which means a structural disorganization of the ECM (Figure 2A) compared to meniscal sample for multi-organ donor in which the stain for collagen fibers is very strong (Figure 2B) as described in Battistelli et al. (18).
The vascularization appears modified, with a low number of blood vessels also in the peripheral third (19).
Classification
The Watanabe’s classification, proposed in 1969, is the most used (21). It is based on the arthroscopic appearance and stability of the pathological meniscus, identifying three different types. In type I and II, the meniscus appears mechanically stable, uniformly thickened (8 to 10 mm) and block shaped but differ in the amount of tibial plateau they cover (type I: complete coverage of the tibial plateau; type II: >80%). In type III (Wrisberg variant) the meniscus is more normally shaped, but unstable, due to the absence of the peripheral posterior meniscus tibial attachments (coronary ligament or popliteomeniscal fascicles), except for the Wrisberg’s ligament. However, several studies did not identify any case of type 3 DLM in their cohorts, while some authors hypothesized a possible traumatic etiology of the type 3 DLM. Furthermore, in 1998, Monllau et al. added a fourth type: a ring-shaped meniscus (22). Since the Watanabe classification is only descriptive, its use for surgical decision-making or planning is doubtful.
In 2004, Klingele et al. (9) proposed a new arthroscopic classification based on morphology (complete or incomplete), peripheral rim stability (stable or unstable after saucerization) and meniscal tears (present or absent). They found that unstable DLM presented at a significantly younger age and were more common in complete DLM than incomplete DLM.
More recently, Ahn et al. (23) proposed a magnetic resonance imaging (MRI)-based classification in which the DLM was classified into four categories (no shift, anterocentral shift, posterocentral shift, and central shift) based on the concept of “meniscal shift” from peripheral detachment of the DLM. Results showed that shift-type DLM were less frequent, but had a significantly larger number of peripheral tears, and repairs were performed more frequently than in the no-shift-type.
Clinical evaluation
Several studies reported that most of the discoid menisci are asymptomatic and the diagnosis is often occasional during arthroscopy or MRI assessment (24-27). Nonetheless, more recently, Grimm et al. (8) reported a 22.5% rate of asymptomatic DLM in a very large cohort of 223 patients with a confirmed DLM discovered incidentally.
Clinical presentation and symptoms are intermittent and described as vague, with an insidious onset. It mostly depends on patient’s age, meniscal instability, and presence of associated lesions. On physical evaluation, the patient may present with limited excursion at terminal 10°–20° of extension, a bulge along the lateral or medial side of the joint, effusion and pain.
Most of patients with a stable DLM (type I and II) are asymptomatic unless a meniscal tear occurs. Unstable variant (type III) shows a hypermobility of the posterior horn, due to the lack of posterior ligamentous attachment, and can present a visible, palpable, or even audible pop during knee motion, along pain and locking (snapping knee syndrome).
The DLM is vulnerable to tearing because of its morphological and structural characteristics, leading to the manifestation of symptoms, such as pain, snapping, or limited extension.
The widely used classification for meniscal tear location and pattern is the O’Connor classification (28), describing the tear pattern as longitudinal, horizontal, oblique, radial, flap, degenerative and complex. The presence of a degenerated ECM, a disorganized collagen network and lack of vascularization in central portion make the DLM prone to tear, even in minimal or absent trauma. The real injury incidence is difficult to evaluate because asymptomatic patients did not routinely perform images, but several authors reported one or more meniscal tears in more than 70% of DLM studied (24,26,29,30). Differently from normal meniscal tear, in DLM is easy to find complex degenerative lesions such as bucket-handle lesion and inverted-type tear (8,31-34).
Physical examination tests (McMurray, Appley and Thessaly) can help in assessing a meniscal tear with good sensitivity and specificity (35-37).
Since bilateralism is described in 79–95% of cases, the other knee should always be evaluated.
Osteochondritis dissecans (OCD) of the lateral femoral condyle is often associated with DLM. OCD is a focal, idiopathic alteration of subchondral bone that may cause instability and disruption of adjacent articular cartilage. Although the etiology of OCD remains unclear, the biomechanical stress due to the abnormal contact force of DLM onto the femoral lateral condyle, may play an important role leading to this disease (38,39). Age, sex, body mass index (BMI), valgus malalignment and type C meniscal shift of the DLM as shown by MRI, are proved to be predisposing factor (25,40,41).
Imaging
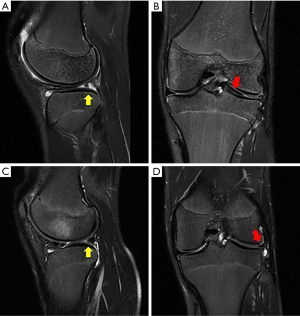
In DLM suspicion, the first step in imaging diagnosis is a plain radiograph, that is usually normal, but it is useful to exclude other conditions, like fractures, OCD and tumors. Radiographs may show indirect signs of DLM, such as block-shape femoral condyle (“squaring”), increased concavity of the tibial plateau, widened joint space, hypoplasia of the lateral tibial spine and meniscal calcifications, increased lateral condyle convex angle (42-44).
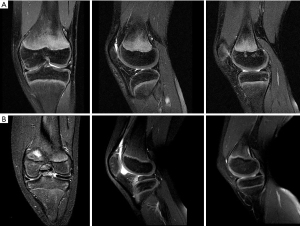
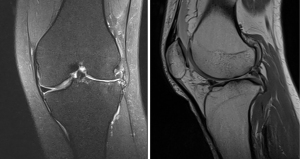
MRI is the modality of choice for evaluating a patient with suspected DLM (Figure 3). MRI is also useful to define the meniscal shape, associated tears and instability (45). In 1989 Silverman (46) proposed five MRI diagnostic criteria for DM: (I) three or more 5-mm slices with continuity between the anterior and posterior horns (bow-tie sign); (II) augmented upper-lower height in the mid-zone generating a bow-tie shape in the sagittal view; (III) differences in size between the anterior and posterior horn, which are usually symmetrical. Additionally, coronal sections show (IV) a complete meniscus in all sections from anterior to posterior through the knee, which is normally only present in the anterior and posterior sections; and (V) an increase in transverse diameter ≤15 mm or ≤20% of the total tibial width (Figure 4). Wrisberg variant (Watanabe’s type III) may show subtle anterior subluxation of the posterior horn, with high T2 signal interposed between the posterior horn and capsule, using MRI assessment.
Treatment
Surgical indication for DLM is usually dependent by clinical presentation. “Wait-and-see” strategy, with no active management, is recommended, in case of incidentally discovered DLM, or in case of asymptomatic DLM, without painful, snapping or locking knee (47).
Surgical treatment should be considered in children that complain knee pain, troublesome sensation of popping or catching during motion or episodes of locking during activities.
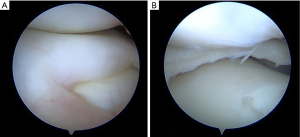
Historically, total meniscectomy has been generally practiced in order to remove completely the cause of discomfort for the child and to prevent possible risks of relapse (48,49). Since total meniscectomy has shown high risk of lateral compartment osteoarthritis and poor clinical outcomes in long-term follow-up (50-52), currently, partial meniscectomy has become the treatment of choice for DLM (25,44,51,53-55). Partial meniscectomy (namely “Centralization” or “Saucerization”) aims to reshape the DLM into a more “normal” “C” configuration, by removing the excess part of the DLM, eliminating possible tears, and assessing, at the same time, potential peripheral rim instabilities (Figures 5,6). The procedure starts with a diagnostic knee arthroscopy through the standard antero-medial and antero-lateral portals. A thoughtful probing is done to search the meniscus type and tear localization. Then, the central portion of the meniscus is removed together with the torn unstable part through arthroscopic punch, scissor and shaver, in order to leave a stable rim from the peripheral capsular attachment.
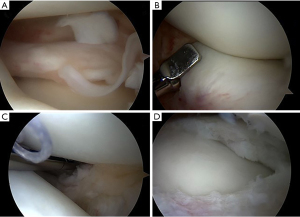
After saucerization, the peripheral rim is inspected for additional tears. If the peripheral rim is teared or unstable, several authors perform a suture repair (52-54,56). An inside-out or all-inside repair usually is effective to stabilize the posterior and middle portion of the rim, while an unstable anterior part is managed by an outside-in suture (Figure 7).
However, despite the shape of the DLM immediately following a partial meniscectomy with repair resembles that of a normal meniscus, a deformation and extrusion of DLM could be seen after surgery in follow-up’s MRI (57-60). In order to preserve DLM shape, Kinugasa et al. (61) proposed a meniscal repair without saucerization. In a small case series of in four patients with painful peripheral longitudinal tear with an intact DLM body, good clinical outcome was observed at 2 years follow-up.
Recently, Saavedra et al. (62) proposed a treatment algorithm for symptomatic DLM: (I) observation and diagnosis of the meniscus shape (complete vs. incomplete), stability, and associated tears; (II) meniscal carving, seeking to preserve the greatest amount of meniscus and emulating a normal meniscus shape; (III) repair with sutures those tears that are amenable; (IV) confirm the peripheral stability of the meniscus, and fix it if unstable.
A major collateral event after surgery of DLM is the developing of OCD (41,63). Mochizuki et al. (41) took in consideration several variables related to OCD but, thanks to multivariate analysis, two elements were found significant: age at surgery and minimum width of the meniscus. This result shows how a younger age and shorter meniscus is linked to an increased risk of OCD. The size of a knee joint varies according to age, body composition, and sex. Ideally, the meniscal volume should be assessed using the standardized volume relative to knee size. The authors demonstrated that the actual and standardized meniscal widths of 7.0 mm and 8.0%, respectively, did not produce osteochondritis lesions.
To date, increasing interest is mounting for meniscus allograft transplantation after DLM, especially when a total or subtotal meniscectomy is required for severe meniscal lesions. This technique has shown initial successful results in older patients with sequelae of total or partial meniscectomy due to DLM (64-67). Kocher et al. (68) reported a case history of three children treated for meniscus transplantation at minimum 2 years follow-up, two of them with DLM. The procedure was accomplished by creating a slot in the tibia, which received the allograft that maintained a peripheral bone block to enhance graft integration. The passing suture was used to stabilize the meniscus, followed by a series of inside-out and outside-in sutures along the full circumference of the meniscus. At follow-up, all three cases, showed successful integration of the allograft, with satisfactory clinical outcomes and no signs of limb discrepancy or angular deformity.
Discussion
In the last decade numerous studies on DLM have been published. We have provided a comprehensive overview of the current state of knowledge on this issue. DLM is a pathology with an underestimate incidence: even if it is estimated about 0.4–17%, the real prevalence is unknown since this variant is often asymptomatic (27). Multiple studies have shown that DLM patients may suffer from early osteoarthritis. Therefore, an early diagnosis and appropriate treatment may delay the degeneration. Currently, the best surgical solution seems to be the “saucerization”, which aims to reshape the DLM removing the excess part of the DLM, eliminating possible tears, and assessing, at the same time, potential peripheral rim instabilities. Several authors questioned if a total or partial meniscectomy could bring to an increased risk of osteoarthritis at long-term follow-up. A recent systematic review established that partial meniscectomy in DLM is more effective in preserving cartilage status, compared with total meniscectomy (87.4% vs. 55.6% of normal or nearly normal cartilage status at follow-up) (47). However, the authors could not demonstrate that these better radiographic results were associated with statistically significant differences in clinical outcomes between the two surgical approaches. In particular, partial meniscectomy still achieves better results (81% rate of good to excellent clinical outcomes, compared to 66.3% rate of excellent results after total meniscectomy), but this difference did not achieve statistically significance. This discrepancy between clinical and radiographic outcomes, could be explained by the relatively young age of patients, that could be more tolerable to pain, because of their better muscular protection around the knee. Another potential explanation was provided by a kinematic study, showing that the maximum lateral tibial translation and maximum internal tibial rotation in the knees with DLM were significantly decreased compared to normal lateral meniscus tear (69). This could be unchanged after surgery, with a subsequent decreased risk of joint pain. Finally, partial or subtotal meniscectomy could correct the varus alignment typical of a DLM knee, reducing the stress on the joint surface (70). On the other hand, no difference in terms of outcome were found between the saucerization alone and the procedure associated with suture repair (55,71); therefore, more consistent studies are necessary to state the need for meniscal suture in DLM, even considering cost-effectiveness. Concerning the risk of osteoarthritis after DLM surgery, Ahn et al. (72) conducted an extended analysis of several factors influencing the onset of osteoarthritis after partial meniscectomy. The authors demonstrated that horizontal tear, prolonged symptom duration, and increased relative meniscal thickness were significant risk factors for radiographic progression to high grade osteoarthritis at a minimum follow-up of 5 years. In his paper, Lee et al. (73) showed that arthroscopic partial meniscectomy performed in young patients with symptomatic DLM, led to unfavorable clinical outcomes in more than 30% of the population enrolled at a mean 10 years follow-up. The OA degeneration progressed in the lateral compartment and the worse outcome seemed to be related to durations of symptoms prior to surgery. Ohnishi et al. (55) compared a standard arthroscopic isolated saucerization or saucerization with suture repair and/or centralization for discoid meniscus in patients aged <13 years with patients aged ≥13 years. Both techniques were effective in improving knee function and preventing early degenerative changes during the short-term follow-up period, but younger group showed a better clinical outcome than the older one, suggesting that age at surgery could be an important factor in reaching good final outcomes. Unfortunately, the development of OA seems related not only to the different qualities (19), but also to the extrusion grade of the DLM (58). In his case series, Koga et al. showed that arthroscopic centralization of the lateral meniscus can possibly prevent progression of OA. The centralized meniscus could function as a “cushion”, averting subsequent extrusion (74,75). Nonetheless, more prolonged follow-up and larger case series are required to assess the real efficacy of these procedure, when performed in very young patients, even considering return to strenuous and competitive sport activities (76).
A limitation of this review is its narrative approach. No meta-analysis of the included articles was performed. Furthermore, as our search was only conducted in one database, relevant articles may have been missed. Nevertheless, we assume this narrative review presents a comprehensive overview of the current state of knowledge of DLM.
Conclusions
DLM is one of the most frequent congenital anomalies of the knee encountered during childhood, and one of the main reasons for consulting orthopedic surgeons about the possibility of surgical correction. Children with incidental found or asymptomatic should be recommended for “wait and see” strategy, however informing parents that DLM could be more susceptible to complex tears. Symptomatic painful DLM should be addressed surgically, restoring typical anatomy using saucerization, tear repair, and stable fixation of the meniscus. The risk of osteoarthritis progression seems to be higher in children with operated DLM, imposing prolonged follow-up and cartilage preserving strategies for these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “The Lateral Meniscus”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-21-31/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-21-31/coif). The series “The Lateral Meniscus” was commissioned by the editorial office without any funding or sponsorship. AG served as the unpaid Guest Editor of the series. SZ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from March 2021 to February 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young R. The external semilunar cartilage as a complete disc. In: Cleland J, Macke J, Young R. editors. Memoirs and Memoranda in Anatomy. London: Williams and Norgate, 1887:179-87.
- Jones RW. Specimen of Internal Semilunar Cartilage as a Complete Disc. Proc R Soc Med 1930;23:1588-9. [Crossref] [PubMed]
- Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am 1982;64:1068-73. [Crossref] [PubMed]
- SMILLIE IS. The congenital discoid meniscus. J Bone Joint Surg Br 1948;30B:671-82. [PubMed]
- Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop Relat Res 1982;19-28. [Crossref] [PubMed]
- Kim SJ, Lee YT, Kim DW. Intraarticular anatomic variants associated with discoid meniscus in Koreans. Clin Orthop Relat Res 1998;202-7. [Crossref] [PubMed]
- Papadopoulos A, Karathanasis A, Kirkos JM, et al. Epidemiologic, clinical and arthroscopic study of the discoid meniscus variant in Greek population. Knee Surg Sports Traumatol Arthrosc 2009;17:600-6. [Crossref] [PubMed]
- Grimm NL, Pace JL, Levy BJ, et al. Demographics and Epidemiology of Discoid Menisci of the Knee: Analysis of a Large Regional Insurance Database. Orthop J Sports Med 2020;8:2325967120950669. [Crossref] [PubMed]
- Klingele KE, Kocher MS, Hresko MT, et al. Discoid lateral meniscus: prevalence of peripheral rim instability. J Pediatr Orthop 2004;24:79-82. [Crossref] [PubMed]
- Bae JH, Lim HC, Hwang DH, et al. Incidence of bilateral discoid lateral meniscus in an Asian population: an arthroscopic assessment of contralateral knees. Arthroscopy 2012;28:936-41. [Crossref] [PubMed]
- Ahn JH, Lee SH, Yoo JC, et al. Bilateral discoid lateral meniscus in knees: evaluation of the contralateral knee in patients with symptomatic discoid lateral meniscus. Arthroscopy 2010;26:1348-56. [Crossref] [PubMed]
- Bedoya MA, Barrera CA, Chauvin NA, et al. Normal meniscal dimensions at different patient ages-MRI evaluation. Skeletal Radiol 2019;48:595-603. [Crossref] [PubMed]
- Fujishiro H, Tsukada S, Nakamura T, et al. Attachment area of fibres from the horns of lateral meniscus: anatomic study with special reference to the positional relationship of anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 2017;25:368-73. [Crossref] [PubMed]
- Nasu H, Nimura A, Sugiura S, et al. An anatomic study on the attachment of the joint capsule to the tibia in the lateral side of the knee. Surg Radiol Anat 2018;40:499-506. [Crossref] [PubMed]
- Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health 2012;4:340-51. [Crossref] [PubMed]
- Andrish JT. Meniscal Injuries in Children and Adolescents: Diagnosis and Management. J Am Acad Orthop Surg 1996;4:231-7. [Crossref] [PubMed]
- CHEN YF. Discoid lateral meniscus of the knee joint. Taiwan Yi Xue Hui Za Zhi 1961;60:154-61. [PubMed]
- Battistelli M, Favero M, Burini D, et al. Morphological and ultrastructural analysis of normal, injured and osteoarthritic human knee menisci. Eur J Histochem 2019;63:2998. [Crossref] [PubMed]
- Bisicchia S, Botti F, Tudisco C. Discoid lateral meniscus in children and adolescents: a histological study. J Exp Orthop 2018;5:39. [Crossref] [PubMed]
- Roach HI, Aigner T, Kouri JB. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis 2004;9:265-77. [Crossref] [PubMed]
- Newman PH. Atlas of Arthroscopy. 2nd edition. Tokyo, Japan: Igaku- Shoin Ltd., 1970.
- Monllau JC, León A, Cugat R, et al. Ring-shaped lateral meniscus. Arthroscopy 1998;14:502-4. [Crossref] [PubMed]
- Ahn JH, Shim JS, Hwang CH, et al. Discoid lateral meniscus in children: clinical manifestations and morphology. J Pediatr Orthop 2001;21:812-6. [Crossref] [PubMed]
- Rohren EM, Kosarek FJ, Helms CA. Discoid lateral meniscus and the frequency of meniscal tears. Skeletal Radiol 2001;30:316-20. [Crossref] [PubMed]
- Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop 2007;1:89-96. [Crossref] [PubMed]
- Sabbag OD, Hevesi M, Sanders TL, et al. Incidence and Treatment Trends of Symptomatic Discoid Lateral Menisci: An 18-Year Population-Based Study. Orthop J Sports Med 2018;6:2325967118797886. [Crossref] [PubMed]
- Kato Y, Oshida M, Aizawa S, et al. Discoid lateral menisci in Japanese cadaver knees. Mod Rheumatol 2004;14:154-9. [Crossref] [PubMed]
- Scranton PE. O’Connorʼs Textbook of Arthroscopic Surgery. 2nd edition. Philadelphia: J.B. Lippincott, 1993.
- Bin SI, Kim JC, Kim JM, et al. Correlation between type of discoid lateral menisci and tear pattern. Knee Surg Sports Traumatol Arthrosc 2002;10:218-22. [Crossref] [PubMed]
- Masquijo JJ, Bernocco F, Porta J. Discoid meniscus in children and adolescents: Correlation between morphology and meniscal tears. Rev Esp Cir Ortop Traumatol (Engl Ed) 2019;63:24-8. [Crossref] [PubMed]
- Servien E, Acquitter Y, Hulet C, et al. Lateral meniscus lesions on stable knee: a prospective multicenter study. Orthop Traumatol Surg Res 2009;95:S60-4. [Crossref] [PubMed]
- Shimozaki K, Nakase J, Takata Y, et al. The characteristic findings of an inverted-type discoid lateral meniscus tear: a hidden tear pattern. BMC Musculoskelet Disord 2019;20:223. [Crossref] [PubMed]
- LaMont L, Ellis H, Wise K, et al. The Inverted Discoid Meniscus Segment: Clinical, Radiographic, and Arthroscopic Description of a Hidden Tear Pattern. Am J Sports Med 2016;44:1534-9. [Crossref] [PubMed]
- Canton G, Maritan G, Impellizzeri F, et al. Bucket-handle meniscal tears in children under the age of 10: a literature review. Acta Biomed 2021;92:e2021020. [PubMed]
- Hegedus EJ, Cook C, Hasselblad V, et al. Physical examination tests for assessing a torn meniscus in the knee: a systematic review with meta-analysis. J Orthop Sports Phys Ther 2007;37:541-50. [Crossref] [PubMed]
- Harrison BK, Abell BE, Gibson TW. The Thessaly test for detection of meniscal tears: validation of a new physical examination technique for primary care medicine. Clin J Sport Med 2009;19:9-12. [Crossref] [PubMed]
- Blyth M, Anthony I, Francq B, et al. Diagnostic accuracy of the Thessaly test, standardised clinical history and other clinical examination tests (Apley's, McMurray's and joint line tenderness) for meniscal tears in comparison with magnetic resonance imaging diagnosis. Health Technol Assess 2015;19:1-62. [Crossref] [PubMed]
- Wei X, Räsänen T, Messner K. Maturation-related compressive properties of rabbit knee articular cartilage and volume fraction of subchondral tissue. Osteoarthritis Cartilage 1998;6:400-9. [Crossref] [PubMed]
- Mitsuoka T, Shino K, Hamada M, et al. Osteochondritis dissecans of the lateral femoral condyle of the knee joint. Arthroscopy 1999;15:20-6. [Crossref] [PubMed]
- Takigami J, Hashimoto Y, Tomihara T, et al. Predictive factors for osteochondritis dissecans of the lateral femoral condyle concurrent with a discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc 2018;26:799-805. [Crossref] [PubMed]
- Mochizuki T, Tanifuji O, Sato T, et al. Predictive factors for developing osteochondritis dissecans after surgery for discoid lateral meniscus are younger age and shorter meniscal width. Knee Surg Sports Traumatol Arthrosc 2021;29:100-8. [Crossref] [PubMed]
- Lu X, Qian J, Yang B, et al. A new radiographic finding of adult symptomatic discoid lateral meniscus. Medicine (Baltimore) 2020;99:e19646. [Crossref] [PubMed]
- Jiang W, Li X, Su H, et al. A new method to diagnose discoid lateral menisci on radiographs. Knee Surg Sports Traumatol Arthrosc 2016;24:1519-24. [Crossref] [PubMed]
- Rao SK, Sripathi Rao P. Clinical, radiologic and arthroscopic assessment and treatment of bilateral discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc 2007;15:597-601. [Crossref] [PubMed]
- Hamada M, Shino K, Kawano K, et al. Usefulness of magnetic resonance imaging for detecting intrasubstance tear and/or degeneration of lateral discoid meniscus. Arthroscopy 1994;10:645-53. [Crossref] [PubMed]
- Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology 1989;173:351-4. [Crossref] [PubMed]
- Kushare I, Klingele K, Samora W. Discoid Meniscus: Diagnosis and Management. Orthop Clin North Am 2015;46:533-40. [Crossref] [PubMed]
- Aichroth PM, Patel DV, Marx CL. Congenital discoid lateral meniscus in children. A follow-up study and evolution of management. J Bone Joint Surg Br 1991;73:932-6. [Crossref] [PubMed]
- Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am 1995;77:1357-61. [Crossref] [PubMed]
- Kim SJ, Chun YM, Jeong JH, et al. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc 2007;15:1315-20. [Crossref] [PubMed]
- Stilli S, Marchesini Reggiani L, Marcheggiani Muccioli GM, et al. Arthroscopic treatment for symptomatic discoid lateral meniscus during childhood. Knee Surg Sports Traumatol Arthrosc 2011;19:1337-42. [Crossref] [PubMed]
- Lee YS, Teo SH, Ahn JH, et al. Systematic Review of the Long-term Surgical Outcomes of Discoid Lateral Meniscus. Arthroscopy 2017;33:1884-95. [Crossref] [PubMed]
- Ahn JH, Kim KI, Wang JH, et al. Long-term results of arthroscopic reshaping for symptomatic discoid lateral meniscus in children. Arthroscopy 2015;31:867-73. [Crossref] [PubMed]
- Atanda A Jr, Wallace M, Bober MB, et al. Arthroscopic Treatment of Discoid Lateral Meniscus Tears in Children With Achondroplasia. J Pediatr Orthop 2016;36:e55-8. [Crossref] [PubMed]
- Ohnishi Y, Nakashima H, Suzuki H, et al. Arthroscopic treatment for symptomatic lateral discoid meniscus: The effects of different ages, groups and procedures on surgical outcomes. Knee 2018;25:1083-90. [Crossref] [PubMed]
- Wasser L, Knörr J, Accadbled F, et al. Arthroscopic treatment of discoid meniscus in children: clinical and MRI results. Orthop Traumatol Surg Res 2011;97:297-303. [Crossref] [PubMed]
- Matsuo T, Kinugasa K, Sakata K, et al. Post-operative deformation and extrusion of the discoid lateral meniscus following a partial meniscectomy with repair. Knee Surg Sports Traumatol Arthrosc 2017;25:390-6. [Crossref] [PubMed]
- Ozeki N, Muneta T, Kawabata K, et al. Centralization of extruded medial meniscus delays cartilage degeneration in rats. J Orthop Sci 2017;22:542-8. [Crossref] [PubMed]
- Lee CR, Bin SI, Kim JM, et al. Magnetic Resonance Imaging Findings in Symptomatic Patients After Arthroscopic Partial Meniscectomy for Torn Discoid Lateral Meniscus. Arthroscopy 2016;32:2366-72. [Crossref] [PubMed]
- Nishino K, Hashimoto Y, Tsumoto S, et al. Morphological Changes in the Residual Meniscus After Reshaping Surgery for a Discoid Lateral Meniscus. Am J Sports Med 2021;49:3270-8. [Crossref] [PubMed]
- Kinugasa K, Hamada M, Yonetani Y, et al. Discoid lateral meniscal repair without saucerization for adolescents with peripheral longitudinal tear. Knee 2019;26:803-8. [Crossref] [PubMed]
- Saavedra M, Sepúlveda M, Jesús Tuca M, et al. Discoid meniscus: current concepts. EFORT Open Rev 2020;5:371-9. [Crossref] [PubMed]
- Yonetani Y, Nakamura N, Natsuume T, et al. Histological evaluation of juvenile osteochondritis dissecans of the knee: a case series. Knee Surg Sports Traumatol Arthrosc 2010;18:723-30. [Crossref] [PubMed]
- Yoon KH, Lee SH, Park SY, et al. Meniscus allograft transplantation for discoid lateral meniscus: clinical comparison between discoid lateral meniscus and nondiscoid lateral meniscus. Arthroscopy 2014;30:724-30. [Crossref] [PubMed]
- Ramme AJ, Strauss EJ, Jazrawi L, et al. Cost effectiveness of meniscal allograft for torn discoid lateral meniscus in young women. Phys Sportsmed 2016;44:278-82. [Crossref] [PubMed]
- Wang SI. Meniscal allograft transplantation for symptomatic knee after meniscectomy of torn discoid medial meniscus: Report of three cases. Acta Orthop Traumatol Turc 2018;52:70-4. [Crossref] [PubMed]
- Zaffagnini S, Espinosa M, Neri MP, et al. Treatment of Meniscal Deficiency with Meniscal Allograft Transplantation and Femoral Osteotomy in a Patient with History of Lateral Discoid Meniscus: 15-Year Follow-up Case Report. JBJS Case Connect 2020;10:e0079. [Crossref] [PubMed]
- Kocher MS, Tepolt FA, Vavken P. Meniscus transplantation in skeletally immature patients. J Pediatr Orthop B 2016;25:343-8. [Crossref] [PubMed]
- Lin Z, Huang W, Ma L, et al. Kinematic features in patients with lateral discoid meniscus injury during walking. Sci Rep 2018;8:5053. [Crossref] [PubMed]
- Kim SJ, Bae JH, Lim HC. Does torn discoid meniscus have effects on limb alignment and arthritic change in middle-aged patients? J Bone Joint Surg Am 2013;95:2008-14. [Crossref] [PubMed]
- Smuin DM, Swenson RD, Dhawan A. Saucerization Versus Complete Resection of a Symptomatic Discoid Lateral Meniscus at Short- and Long-term Follow-up: A Systematic Review. Arthroscopy 2017;33:1733-42. [Crossref] [PubMed]
- Ahn JH, Kang DM, Choi KJ. Risk factors for radiographic progression of osteoarthritis after partial meniscectomy of discoid lateral meniscus tear. Orthop Traumatol Surg Res 2017;103:1183-8. [Crossref] [PubMed]
- Lee CR, Bin SI, Kim JM, et al. Arthroscopic partial meniscectomy in young patients with symptomatic discoid lateral meniscus: an average 10-year follow-up study. Arch Orthop Trauma Surg 2018;138:369-76. [Crossref] [PubMed]
- Koga H, Muneta T, Yagishita K, et al. Arthroscopic centralization of an extruded lateral meniscus. Arthrosc Tech 2012;1:e209-12. [Crossref] [PubMed]
- Koga H, Muneta T, Watanabe T, et al. Two-Year Outcomes After Arthroscopic Lateral Meniscus Centralization. Arthroscopy 2016;32:2000-8. [Crossref] [PubMed]
- Grassi A, Bailey JR, Filardo G, et al. Return to Sport Activity After Meniscal Allograft Transplantation: At What Level and at What Cost? A Systematic Review and Meta-analysis. Sports Health 2019;11:123-33. [Crossref] [PubMed]
Cite this article as: Trisolino G, Stallone S, Grassi A, Olivotto E, Battistelli M, Zarantonello P, Gallone G, Ferrari D, Di Gennaro GL, Zaffagnini S. The discoid lateral meniscus in children: a narrative review of pathology, diagnosis and treatment. Ann Joint 2022;7:38.


