
Radiofrequency ablation prior to total knee arthroplasty does not improve post-surgical pain or recovery: a double-blinded, multi-center, randomized clinical trial
Highlight box
Key findings
• Radiofrequency ablation of the genicular nerves prior to TKA did not affect opioid use or cessation, pain levels, or WOMAC scores following TKA.
What is known and what is new?
• Radiofrequency ablation targeting the genicular nerves has been used an effective treatment for knee pain in the nonoperative management of knee osteoarthritis.
• This manuscript adds new information about radiofrequency ablation for clinicians seeking pre-operative interventions for their patients who are planning to undergo TKA.
What is the implication, and what should change now?
• The current techniques of traditional RFA and cooled RFA of the geniculate nerves used in this study are not indicated as routine preoperative interventions to improve short-term surgical recovery after TKA.
Introduction
By 2030, it is projected that there will be 935,000 total knee arthroplasties (TKA) performed in the United States, with subsequent increases expected (1). The number of patients experiencing symptomatic knee osteoarthritis (OA) may currently be as high as 14 million, many of whom are younger than 65 years of age (2).
TKA remains a cost-effective approach to treating knee OA. The procedure, however, is often associated with a prolonged recovery. The risks of complications and costs associated with convalescence are significant. There is an established need for optimizing recovery and reducing risks associated with knee arthroplasty (3,4).
Many of the medical complications associated with TKA are related to the postoperative use of opioid analgesic medications, including nausea, constipation, urinary retention, malaise, mental status changes, abuse, and addiction (5,6). Therefore, the development of pain management protocols that decrease the use of opioids in TKA is currently a common goal in the field of orthopedic surgery. There is a need for studies evaluating the efficacy of alternative non-opioid approaches to pain management in orthopedics (7).
The use of thermal peripheral sensory nerve ablation, also known as radiofrequency ablation (RFA), for treating regional pain has increased in the past decade. According to Ball (8), this technique exploits current anatomic knowledge of terminal afferent-only nerve endings allowing for a targeted approach to disrupt the transmission of noxious stimuli from those endings with minimally invasive techniques. In the traditional version of this technology (t-RFA), a radiofrequency probe is inserted into the anatomic location of the nerve, the tissue is heated, and the result is reversible axonal damage. A more technical approach, cooled RFA (c-RFA), protects the tissues immediately adjacent to the probe tip from excessive heating, allowing for a larger area of nerve ablation (9). Because the anatomic course of the geniculate nerves is varied and the probe can be positioned based on fluoroscopic landmarks, c-RFA would theoretically be more likely to achieve neurotomy than t-RFA (10).
Since most pain from the knee joint is thought to be experienced by transmission from geniculate afferent branches, it has been hypothesized that preoperative neurotomy of the terminal endings of those nerves might reduce surgical and postsurgical pain (11,12), thereby improving other aspects of recovery from TKA, including pain scores, opioid usage, and validated functional scores.
In the current study, geniculate nerves targeted included the superior medial, inferior medial, and middle genicular nerves (branches of the tibial nerve), as well as the superior lateral genicular nerve (a branch of the femoral nerve). Prior studies have shown targeting these nerves with RFA techniques reduced pain associated with knee OA (13,14).
The purpose of this randomized clinical trial was to determine the effects of c-RFA and t-RFA on three-month clinical measures after TKA. We compared c-RFA and t-RFA to one another and to a sham procedure. We hypothesized that both t-RFA and c-RFA, compared to sham procedure, would reduce pain, opioid usage, time to opioid cessation, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the short term (3 months) after TKA, with c-RFA having a more significant impact. To detect any unexpected complications or side effects from the procedure we followed patients for a year after TKA. This study was conducted in accordance with the CONSORT reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-33/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Providence Hospital Institutional Review Board, Spokane, Washington (IRB No. 00000800), and informed consent was taken from all the patients. The study was conducted in the United States as a multicenter, double-blinded, parallel-group trial with balanced randomization (1:1:1).
The study was powered based on expected changes in pain scores. An ad hoc power analysis was performed with a commonly used nomogram (15). Assuming a standardized difference of 0.82 (for pain scores a clinically significant difference of 2 was assumed and based on prior RFA studies for OA a standard deviation of 2.4 was used), a power of 0.80, and an alpha level of 0.05, we determined a sample size of 45 (n=45) to be needed for each arm.
The study was conducted at The Institute for Orthopedic Research and Innovation, Coeur d'Alene, Idaho. Study interventions were performed at Pleasant View Surgery Center, Post Falls, Idaho. Surgical (TKA) procedures were performed at Northwest Specialty Hospital, Post Falls, Idaho, and Providence Sacred Heart Hospital, Spokane, Washington.
Participants and enrollment
Between February 2017 and November 2018, a total of 207 participants were prescreened for the study, according to the inclusion and exclusion criteria (Table 1). One hundred forty-three candidates met the inclusion/exclusion criteria and were enrolled by one of three different orthopedic surgeons in this trial (Table 1). Enrolled participants were then randomized to one of three pain management providers using a random number generator (numbergenerator.org). Two of the three pain management providers had significant clinical experience with both t-RFA and c-RFA in the knee, and the third had significant experience with t-RFA only in the knee prior to study commencement. All pain management providers underwent standardized pre-study training by the manufacturer of the RFA devices (Avanos Medical, Alpharetta, GA, USA) to ensure consistent technique.
Table 1
| Inclusion criteria |
| Ages 45–80 years |
| Osteoarthritis of the knee where unilateral TKA is clinically indicated |
| Readiness to undergo study protocol including c-RFA/t-RFA and sham procedure |
| Body mass index: <40 kg/m2 |
| Primary TKA |
| Exclusion criteria |
| No daily opioid use 5 weeks prior to enrollment |
| No documented narcotic dependency or recreational drug use |
| No tobacco usage within 2 months prior to surgery |
| No confounding inflammatory arthritis disease |
| No neuropathy/neurologic impairment |
| No significant acute illness |
| No other confounding chronic pain |
| No investigational drug within 3 months prior to enrollment |
| No diagnosed thrombophilia/bleeding disorders |
| No severe cardio-pulmonary conditions |
| No allergic reaction to local anesthetics or implants |
| No breastfeeding/pregnancy |
| No confounding psychiatric illness |
| No confounding significant concurrent hardware removal |
TKA, total knee arthroplasty; c-RFA, cooled radiofrequency ablation; t-RFA, traditional radiofrequency ablation.
All participants were blinded to their designated treatment groups. All participants received RFA or sham at the same facility on an outpatient basis under conscious sedation. All RFA/sham procedures were performed 3–12 weeks before their planned TKA. Since RFA is performed with needle-sized probes, it does create minor cutaneous lesions and it was the surgeons’ preferences that these lesions be completely resolved prior to TKA surgery. Therefore, we chose a minimum of 3 weeks between RFA and knee arthroplasty. Fifty-nine participants received their procedure 3–4 weeks prior to surgery, 66 received their procedure 5–8 weeks prior to surgery, and 14 participants received their procedure 9–12 weeks prior to surgery.
Any subject requiring a delay between the intervention and TKA past 12 weeks was withdrawn from the study (n=4).
TKA surgeries were performed by one of three referring high-volume orthopedic surgeons. All three surgeons performed TKA surgery according to their traditional techniques and preferences; two of the three surgeons preferred cruciate-retaining arthroplasty technique while the other preferred a posterior stabilized technique. All knees in this study underwent patella resurfacing.
Anesthesia care during TKA was determined by anesthesiologist and surgeon preferences and consisted of 28/139 (20.1%) participants receiving a spinal anesthetic and 111/139 (79.9%) receiving a general anesthetic augmented by a periarticular infiltration of local anesthetic. Eighteen participants (18/139, 12.9%) received a single shot (adductor canal) nerve block. Peri- and post-operative analgesics were prescribed in a standardized manner to all participants including oral and IV opioids with a pain-dependent protocol.
Participants were discharged on days 0–3 and referred to a preapproved, enrolled physical therapy office with a universal study protocol for rehabilitation. The protocol involved early weightbearing and ambulation, focused on early progression range of motion, and included a standardized approach for all participants.
For one year following TKA, participants were followed at important time points (postoperative day 3, week 1, week 2, week 12, month 6, and month 12) using phone interviews and routine office visits. The primary endpoint on which the study was designed and powered was three months following TKA.
Data collection
All nursing staff, research staff, and surgeons were blinded with respect to intervention type until data collection was completed for each participant.
Participant baseline numeric pain scores and WOMAC scores were initially recorded by research staff on the date of enrollment in the study, prior to scheduling the RFA procedure.
To evaluate any side effects from the RFA/sham interventions, we recorded any minor side effects, including bruising, swelling, and pain/discomfort. We also collected adverse events such as infection or skin numbness.
Pre-TKA (after study intervention but prior to TKA) pain scores and WOMAC scores were recorded at a routine pre-TKA surgeon office visit. All data at this visit was collected independently and in a blinded fashion by research staff.
All inpatient pain scores and narcotic usage (both orally and intravenously administered) were obtained by review of hospital records by research staff and converted to daily oral morphine equivalents (MEQ) (16).
Follow up records of outpatient narcotic usage, pain scores, and WOMAC scores were obtained by research staff by phone interview (postoperative day 3, week 1, week 12, and month 6) and at the time of routine office visit (postoperative week 2, week 6, and month 12). A standardized pain/narcotic use diary was provided to the participants and used to determine accuracy of interviews and time to cessation.
Statistical analysis
All measures are presented based on the subject’s randomization assignment. Continuous data are presented as means, medians, ranges, and 95% confidence intervals; categorical data are presented as counts and percentages. The primary and secondary outcomes and other relevant measures are compared between randomized groups based on one-way analysis of variance (ANOVA), Kruskal-Wallis, or Fisher’s exact tests, as appropriate. All P values are considered significant at the two-sided significance level of 5%. The P values are not adjusted for multiple testing. All statistical analyses are performed using SAS software version 9.4.
Results
A CONSORT diagram is used to comply with standards for reporting prospective trials (Figure 1). This method was chosen because it is endorsed by prominent medical journals and is the evidence-based best practice for reporting randomized control trials (17).
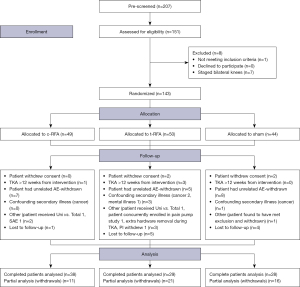
The patient demographics (Table 2), as well as baseline pain, and baseline WOMAC scores (Tables 3,4) demonstrated no statistically significant differences among the three groups.
Table 2
| Baseline characteristics | Sham (n=44) | t-RFA (n=50) | c-RFA (n=49) |
|---|---|---|---|
| Age (years) | |||
| N | 44 | 50 | 49 |
| Mean ± SD | 64.3±8.1 | 64.2±9.3 | 66.9±8.4 |
| Median | 64 | 65.5 | 67 |
| Min., Max. | 50, 80 | 45, 82 | 52, 84 |
| Gender | |||
| N | 44 | 50 | 49 |
| Female, n [%] | 26 [59] | 30 [60] | 25 [51] |
| Male, n [%] | 18 [41] | 20 [40] | 24 [49] |
| Knee | |||
| N | 44 | 50 | 49 |
| Left, n [%] | 25 [57] | 28 [56] | 24 [49] |
| Right, n [%] | 19 [43] | 22 [44] | 25 [51] |
| Number of days from randomization to TKA | |||
| N | 42 | 46 | 48 |
| Mean ± SD | 42.0±21.1 | 42.6±22.6 | 33.3±16.5 |
| Median | 35 | 35 | 29 |
| Min., Max. | 20, 125 | 20, 139 | 20, 119 |
| Missing | 2 | 4 | 1 |
Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation; TKA, total knee arthroplasty; SD, standard deviation.
Table 3
| Variables | NRS values | P value* | ||
|---|---|---|---|---|
| Sham (n=44) | t-RFA (n=50) | c-RFA (n=49) | ||
| Baseline, prior to study intervention | ||||
| N | 44 | 48 | 49 | – |
| Mean ± SD | 4.3±2.2 | 3.6±2.3 | 3.7±2.6 | 0.3988 |
| Median | 4.0 | 3.0 | 4.0 | – |
| Min., Max. | 0.0, 9.0 | 0.0, 8.0 | 0.0, 9.0 | – |
| 95% CI | 3.6, 4.9 | 3.0, 4.3 | 3.0, 4.5 | – |
| Pre-operative, after study intervention | ||||
| N | 39 | 43 | 47 | – |
| Mean ± SD | 2.9±2.0 | 2.6±2.0 | 2.4±2.0 | 0.6124 |
| Median | 2.0 | 2.0 | 2.0 | – |
| Min., Max. | 0.0, 7.0 | 0.0, 8.0 | 0.0, 6.5 | – |
| 95% CI | 2.2, 3.5 | 2.0, 3.2 | 1.9, 3.0 | – |
| 1 week post-operative, phone interview | ||||
| N | 36 | 37 | 44 | – |
| Mean ± SD | 3.8±2.1 | 3.9±1.7 | 3.0±1.6 | 0.0541 |
| Median | 4.0 | 3.5 | 3.0 | – |
| Min., Max. | 0.0, 8.0 | 1.0, 8.5 | 0.0. 6.0 | – |
| 95% CI | 3.1, 4.5 | 3.3, 4.5 | 2.5, 3.5 | – |
| 2 weeks post-operative | ||||
| N | 38 | 42 | 42 | – |
| Mean ± SD | 3.4±2.1 | 3.6±1.8 | 2.8±1.6 | 0.1983 |
| Median | 3.0 | 3.3 | 3.0 | – |
| Min., Max. | 0.0, 10.0 | 0.0, 8.0 | 0.0, 6.0 | – |
| 95% CI | 2.7, 4.0 | 3.0, 4.1 | 2.3, 3.4 | – |
| 6 weeks post-operative | ||||
| N | 37 | 37 | 42 | – |
| Mean ± SD | 1.6±1.5 | 1.9±1.5 | 1.5±1.5 | 0.5474 |
| Median | 1.0 | 2.0 | 1.0 | – |
| Min., Max. | 0.0, 6.0 | 0.0, 5.0 | 0.0, 5.0 | – |
| 95% CI | 1.1, 2.1 | 1.4, 2.4 | 1.1, 2.0 | – |
| 12 weeks post-operative, phone interview | ||||
| N | 33 | 36 | 43 | – |
| Mean ± SD | 0.7±1.0 | 1.3±1.3 | 0.6±1.2 | 0.0597 |
| Median | 0.0 | 1.0 | 0.0 | – |
| Min., Max. | 0.0, 3.5 | 0.0, 4.0 | 0.0, 6.0 | – |
| 95% CI | 0.4, 1.1 | 0.8, 1.7 | 0.2, 1.0 | – |
| 6 months post-operative, phone interview | ||||
| N | 28 | 29 | 38 | – |
| Mean ± SD | 0.3±0.5 | 0.9±1.6 | 0.6±1.4 | 0.1361 |
| Median | 0.0 | 0.0 | 0.0 | – |
| Min., Max. | 0.0, 2.0 | 0.0, 8.0 | 0.0, 6.0 | – |
| 95% CI | 0.1, 0.4 | 0.3, 1.6 | 0.1, 1.1 | – |
| 1 year post-operative | ||||
| N | 19 | 15 | 24 | – |
| Mean ± SD | 0.2±0.5 | 0.3±0.6 | 0.3±0.7 | 0.9614 |
| Median | 0.0 | 0.0 | 0.0 | – |
| Min., Max. | 0.0, 2.0 | 0.0, 2.0 | 0.0, 3.0 | – |
| 95% CI | −0.0, 0.5 | −0.1, 0.6 | −0.0, 0.5 | – |
*, P value is difference in means between groups based on one-way analysis of variance. Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation; CI, confidence interval; NRS, Numeric Rating Scale; SD, standard deviation.
Table 4
| Variables | WOMAC total score | P value* | ||
|---|---|---|---|---|
| Sham (n=44) | t-RFA (n=50) | c-RFA (n=49) | ||
| Baseline, prior to study intervention | ||||
| N | 44 | 47 | 49 | – |
| Mean ± SD | 57.6±17.9 | 51.7±16.8 | 50.7±17.8 | 0.1327 |
| Median | 58.3 | 54.2 | 51.0 | – |
| Min., Max. | 11.5, 100.0 | 13.5, 86.5 | 3.1, 79.7 | – |
| 95% CI | 52.2, 63.0 | 46.7, 56.6 | 45.6, 55.8 | – |
| Pre-operative, after study intervention | ||||
| N | 38 | 42 | 44 | – |
| Mean ± SD | 50.1±17.6 | 43.8±19.0 | 45.9±18.5 | 0.3068 |
| Median | 54.7 | 47.9 | 50.3 | – |
| Min., Max. | 12.5, 94.8 | 14.6, 90.2 | 0.0, 77.1 | – |
| 95% CI | 44.3, 55.9 | 37.9, 49.7 | 40.3, 51.5 | – |
| 2 weeks post-operative | ||||
| N | 38 | 38 | 40 | – |
| Mean ± SD | 50.1±15.6 | 55.0±13.5 | 47.2±16.3 | 0.0754 |
| Median | 51.6 | 55.3 | 47.8 | – |
| Min., Max. | 12.5, 79.2 | 24.0, 80.2 | 9.5, 89.6 | – |
| 95% CI | 44.9, 55.2 | 50.6, 59.5 | 42.0, 52.4 | – |
| 6 weeks post-operative | ||||
| N | 37 | 34 | 41 | – |
| Mean ± SD | 31.1±18.5 | 34.4±17.0 | 28.7±17.4 | 0.3777 |
| Median | 31.3 | 36.4 | 29.3 | – |
| Min., Max. | 5.2, 65.6 | 0.0, 70.8 | 1.0, 75.0 | – |
| 95% CI | 24.9, 37.3 | 28.5, 40.4 | 23.2, 34.2 | – |
| 1 year post-operative | ||||
| N | 19 | 15 | 24 | – |
| Mean ± SD | 9.7±12.0 | 8.6±11.3 | 8.4±11.9 | 0.9344 |
| Median | 7.3 | 4.2 | 3.7 | – |
| Min., Max. | 0.0, 46.7 | 0.0, 35.4 | 0.0, 39.1 | – |
| 95% CI | 3.9, 15.4 | 2.4, 14.8 | 3.4, 13.4 | – |
*, P value is difference in means between groups based on one-way analysis of variance. WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation; CI, confidence interval; SD, standard deviation.
Side effects related to treatment
Intervention side effects were reported in t-RFA (38%), c-RFA (21%), and sham (20%). Adverse events related to the RFA/sham procedure were universally mild. The treatment-related side effects were discomfort (29%, 21%, 20%), swelling (7%, 6%, 2%), and bruising (5%, 2%, 0 %) in t-RFA, c-RFA and sham, respectively. No infections were reported. Complications of numbness were only reported in the t-RFA group (2%). No subject reported “worsened pain” as a result of intervention (Table 5).
Table 5
| Side effects | Sham, n [%] | t-RFA, n [%] | c-RFA, n [%] | |||||
|---|---|---|---|---|---|---|---|---|
| Events | Subjects (n=41) | Events | Subjects (n=42) | Events | Subjects (n=48) | |||
| Total side effects | 151 | 32 [78] | 151 | 37 [88] | 159 | 36 [75] | ||
| Side effects related to intervention | 9 | 8 [20] | 18 | 16 [38] | 13 | 10 [21] | ||
| Pain/discomfort around treated area | 8 | 8 [20] | 12 | 12 [29] | 10 | 10 [21] | ||
| Swelling | 1 | 1 [2] | 3 | 3 [7] | 3 | 3 [6] | ||
| Bruising | 0 | 0 [0] | 2 | 2 [5] | 1 | 1 [2] | ||
| Infection | 0 | 0 [0] | 0 | 0 [0] | 0 | 0 [0] | ||
| Numbness | 0 | 0 [0] | 2 | 1 [2] | 0 | 0 [0] | ||
| Worsened pain | 0 | 0 [0] | 0 | 0 [0] | 0 | 0 [0] | ||
Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation.
Hospital length of stay
There were no significant differences in length of stay between t-RFA (1.7±0.9 days), c-RFA (1.8±1.1 days), and sham (1.6±0.7 days) groups (Table 6) (note that at the time of this study, outpatient TKA was not customary practice for the surgeons participating in this study, and day of surgery discharges were uncommon).
Table 6
| Inpatient measures | Sham (n=44) | t-RFA (n=50) | c-RFA (n=49) | P value |
|---|---|---|---|---|
| Length of stay (days) | ||||
| N | 42 | 42 | 46 | – |
| Mean ± SD | 1.6±0.7 | 1.7±0.9 | 1.8±1.1 | 0.6989* |
| Median | 2.0 | 2 | 2 | – |
| Min., Max. | 0, 3 | 0, 4 | 0, 5 | – |
| 95% CI | 1.4, 1.8 | 1.4, 2.0 | 1.5, 2.1 | – |
| Missing | 2 | 8 | 3 | – |
| Length of stay, n [%] | ||||
| N | 42 | 42 | 46 | – |
| 0 days | 1 [2] | 1 [2] | 4 [9] | 0.8160** |
| 1 day | 19 [45] | 19 [45] | 17 [37] | – |
| 2 days | 17 [40] | 14 [33] | 12 [26] | – |
| 3 days | 5 [12] | 7 [17] | 12 [26] | – |
| 4 days | 0 [0] | 1 [2] | 0 [0] | – |
| 5 days | 0 [0] | 0 [0] | 1 [2] | – |
| Missing | 2 | 8 | 3 | – |
| NRS, inpatient day 1 | ||||
| N | 40 | 39 | 42 | – |
| Mean ± SD | 2.9±1.4 | 3.3±1.8 | 3.2±1.8 | 0.5008* |
| Median | 2.5 | 3.0 | 3.0 | – |
| Min., Max. | 0.7, 7.0 | 0.0, 7.5 | 0.0, 6.5 | – |
| 95% CI | 2.4, 3.4 | 2.7, 3.9 | 2.7, 3.8 | – |
| NRS, inpatient day 2 | ||||
| N | 41 | 41 | 42 | – |
| Mean ± SD | 3.7±1.5 | 3.7±1.4 | 3.2±1.4 | 0.1721* |
| Median | 3.5 | 3.7 | 3.5 | – |
| Min., Max. | 1.3, 8.0 | 1.8, 7.3 | 0.7, 7.2 | – |
| 95% CI | 3.2, 4.2 | 3.3, 4.2 | 2.8, 3.7 | – |
| NRS, inpatient day 3 | ||||
| N | 22 | 22 | 25 | – |
| Mean ± SD | 3.3±1.3 | 3.6±1.5 | 3.3±1.4 | 0.7199* |
| Median | 3.4 | 3.4 | 3.3 | – |
| Min., Max. | 1.0, 7.0 | 1.3, 7.3 | 1.0, 6.3 | – |
| 95% CI | 2.8, 3.9 | 2.9, 4.3 | 2.7, 3.8 | – |
| NRS, inpatient day 4 | ||||
| N | 5 | 8 | 13 | – |
| Mean ± SD | 3.7±0.7 | 2.3±1.6 | 2.6±1.3 | – |
| Median | 4.0 | 2.7 | 2.5 | 0.2043* |
| Min., Max. | 2.5, 4.3 | 0.0, 4.3 | 0.5, 5.0 | – |
| 95% CI | 2.8, 4.6 | 1.0, 3.7 | 1.8, 3.4 | – |
| NRS, inpatient day 5 | ||||
| N | 0 | 1 | 1 | – |
| Mean ± SD | – | 2.3±N/A | 1.3±N/A | N/A |
| Median | – | 2.3 | 1.3 | – |
| Min., Max. | – | 2.3, 2.3 | 1.3, 1.3 | – |
| 95% CI | – | – | – | – |
*, P value is difference in means between groups based on one-way analysis of variance; **, P value is based on Kruskal-Wallis test indicating whether the distributions are the same. Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation; CI, confidence interval; SD, standard deviation; NRS, Numeric Rating Scale (pain); N/A, not available.
Differences in postoperative outcomes
Pain
There were no significant differences in Numeric Rating Scale (NRS) score on any hospital day reported (Table 6). Similarly, there were no differences in reported NRS scores at week 1, week 2, week 6, or week 12 between any of the three groups (Table 3).
Opioid use
Daily inpatient opioid usage is reported in Table 7. For the purposes of reporting, the day of surgery is listed as “inpatient day 1”. There were no differences in daily inpatient opioid use among the three groups. Opioid use peaked for all three groups on the first postoperative day (inpatient day 2) and trended down on subsequent inpatient days.
Table 7
| Opioid use measures | Sham (n=44) | t-RFA (n=50) | c-RFA (n=49) | P value |
|---|---|---|---|---|
| Subjects taking opioids during inpatient days, n [%] | ||||
| N | 44 | 50 | 49 | – |
| Inpatient day 1 | 40 [91] | 40 [80] | 39 [80] | 0.2717*** |
| Inpatient day 2 | 40 [91] | 41 [82] | 42 [86] | 0.4472*** |
| Inpatient day 3 | 22 [50] | 22 [44] | 25 [51] | 0.7597*** |
| Inpatient day 4 | 5 [11] | 8 [16] | 13 [27] | 0.1645*** |
| Inpatient day 5 | 0 [0] | 1 [2] | 2 [4] | 0.6483*** |
| Inpatient day 6 | 0 [0] | 0 [0] | 1 [2] | 0.6503*** |
| MEQ (mg), inpatient day 1 | ||||
| N | 40 | 40 | 39 | – |
| Mean ± SD | 31.4±19.4 | 35.2±16.3 | 30.6±19.7 | 0.4946* |
| Median | 30.0 | 37.5 | 25.0 | – |
| Min., Max. | 5.0, 90.0 | 8.0, 75.0 | 7.5, 97.5 | – |
| 95% CI | 25.2, 37.6 | 30.0, 40.4 | 24.2, 37.0 | – |
| MEQ (mg), inpatient day 2 | ||||
| N | 40 | 41 | 42 | – |
| Mean ± SD | 58.9±30.3 | 62.9±37.0 | 53.9±32.8 | 0.4735* |
| Median | 52.5 | 60.0 | 51.3 | – |
| Min., Max. | 15.0, 142.5 | 7.5, 180.0 | 5.0, 150.0 | – |
| 95% CI | 49.2, 68.6 | 51.3, 74.6 | 43.7, 64.1 | – |
| MEQ (mg), inpatient day 3 | ||||
| N | 22 | 22 | 25 | – |
| Mean ± SD | 49.1±26.3 | 49.9±34.2 | 51.2±35.6 | 0.9749* |
| Median | 41.3 | 48.8 | 45.0 | – |
| Min., Max. | 10.0, 120.0 | 10.0, 135.0 | 5.0, 135.0 | – |
| 95% CI | 37.4, 60.8 | 34.7, 65.0 | 36.5, 65.9 | – |
| MEQ (mg), inpatient day 4 | ||||
| N | 5 | 8 | 13 | – |
| Mean ± SD | 37.0±5.4 | 30.0±24.1 | 37.1±22.3 | – |
| Median | 37.5 | 21.3 | 30.0 | 0.2490** |
| Min., Max. | 30.0, 45.0 | 7.5, 75.0 | 15.0, 90.0 | – |
| 95% CI | 30.3, 43.7 | 9.9, 50.1 | 23.6, 50.6 | – |
| MEQ (mg), inpatient day 5 | ||||
| N | 0 | 1 | 2 | – |
| Mean ± SD | – | 10.0±N/A | 26.3±5.3 | – |
| Median | – | 10.0 | 26.3 | 0.2207** |
| Min., Max. | – | 10.0, 10.0 | 22.5, 30.0 | – |
| 95% CI | – | – | −21.4, 73.9 | – |
| MEQ (mg), inpatient day 6 | ||||
| N | 0 | 0 | 1 | – |
| Mean ± SD | – | – | 7.5±N/A | N/A |
| Median | – | – | 7.5 | – |
| Min., Max. | – | – | 7.5, 7.5 | – |
| 95% CI | – | – | – | – |
*, P value is difference in means between groups based on one-way analysis of variance; **, P value is based on Kruskal-Wallis test indicating whether the distributions are the same; ***, P value is Fisher’s exact test. Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation; MEQ, daily morphine equivalents; CI, confidence interval; SD, standard deviation; N/A, not available.
There were no significant differences in opioid usage among the three groups at any of the follow-up time points. There was no significant difference in the number of participants taking opioids at week 12 between the groups. There were no significant differences in the mean number of days to cessation among the three groups (Table 8).
Table 8
| Opioid use measures | Sham (n=44) | t-RFA (n=50) | c-RFA (n=49) | P value |
|---|---|---|---|---|
| Subjects taking opioids during follow-up phone interviews, n [%] | ||||
| N | 44 | 50 | 49 | – |
| 1 week post-operative | 30 [68] | 35 [70] | 39 [80] | 0.3979*** |
| 12 weeks post-operative | 0 [0] | 5 [10] | 1 [2] | 0.0488*** |
| 6 months post-operative | 0 [0] | 0 [0] | 0 [0] | N/A |
| MEQ (mg), 1 week post-operative, phone interview | ||||
| N | 30 | 35 | 39 | – |
| Mean ± SD | 33.9±21.1 | 42.3±24.3 | 36.5±23.3 | 0.3159* |
| Median | 30.0 | 42.5 | 32.5 | – |
| Min., Max. | 5.0, 77.5 | 5.0, 102.5 | 5.0, 90.0 | – |
| 95% CI | 26.0, 41.8 | 34.0, 50.7 | 28.9, 44.0 | – |
| MEQ (mg), 12 weeks post-operative, phone interview | ||||
| N | 0 | 5 | 1 | – |
| Mean ± SD | – | 8.5±2.2 | 5.0±N/A | – |
| Median | – | 10.0 | 5.0 | 0.2059** |
| Min., Max. | – | 5.0, 10.0 | 5.0, 5.0 | – |
| 95% CI | – | 5.7, 11.3 | – | – |
| MEQ (mg), 6 months post-operative, phone interview | ||||
| N | 0 | 0 | 0 | – |
| Mean ± SD | – | – | – | N/A |
| Median | – | – | – | – |
| Min., Max. | – | – | – | – |
| 95% CI | – | – | – | – |
| Days to cessation | ||||
| N | 37 | 31 | 42 | – |
| Mean ± SD | 32.2±17.9 | 42.6±30.7 | 38.4±22.0 | 0.1905* |
| Median | 33 | 39 | 40 | – |
| Min., Max. | 3, 80 | 3, 168 | 3, 97 | – |
| 95% CI | 26.2, 38.2 | 31.3, 53.8 | 31.6, 45.3 | – |
*, P value is difference in means between groups based on one-way analysis of variance; **, P value is based on Kruskal-Wallis test indicating whether the distributions are the same; ***, P value is Fisher’s exact test. Sham, simulated intervention without ablation; t-RFA, traditional radiofrequency ablation; c-RFA, cooled radiofrequency ablation; MEQ, daily morphine equivalents; CI, confidence interval; SD, standard deviation; N/A, not available.
WOMAC
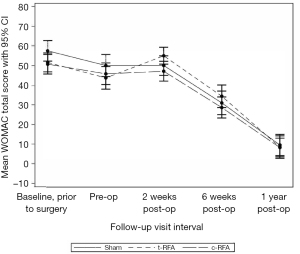
There were no significant differences in WOMAC scores among the three groups at any of the postoperative time points (Figure 2).
Discussion
We investigated RFA technology delivered before TKA for its potential to improve recovery in the short-term after TKA and found no significant improvements among c-RFA, t-RFA, or sham groups. There were no significant differences in terms of hospital length of stay, patient-reported pain scores, opioid usage, or time to opioid cessation. There were also no differences in WOMAC scores between those patients receiving c-RFA, t-RFA, or sham procedures. Adverse effects of the RFA procedures were universally mild.
The results of this study support the findings of similar studies in which no measurable effect of c-RFA on TKA outcomes was found when performed 2–6 weeks before TKA (12,18). One of these studies also indicated no improvement in post-surgical pain at 1, 3, and 6 months postoperatively compared to placebo (12). More recently, Stake et al. (19) reported reductions in the odds of the need for prolonged narcotic use, urinary tract infections, and blood transfusions in patients receiving preoperative RFA compared to a database cohort. Dasa et al. previously reported on the possible benefits of cryoablation of the geniculate nerves prior to TKA (11). Cryoablation differs from RFA in that it uses freezing of geniculate nerves, not heating, to disrupt the function of the nerve. More recently, Mihalko et al. described reductions in opioid use and pain scores, as well as potentially improved functional outcomes, with the use of cryoablation of the geniculate nerves prior to TKA (20).
Prior well-designed trials have reported significant efficacy from t-RFA, and especially c-RFA, in nonoperative management of knee OA pain, as well as a cost-effective benefit with respect to accepted measures (13,21,22). The current study differed from most other RFA studies because this study did not require a minimum pretreatment pain score as part of the inclusion criteria. Therefore, the mean pre-treatment and pre-arthroplasty pain scores were lower than most other RFA studies. The mean pretreatment NRS pain score in this study was between 3.7 and 4.4, which is consistent with other reports of pre-surgical knee OA (23).
There are several potential reasons the results of RFA in this study do not show benefit in TKA as opposed to other studies indicating an improvement in knee OA-related pain. There may be different nerves communicating pain in knee arthroplasty as opposed to knee OA. The capsular branches from the distal sciatic/popliteal nerve are thought to provide significant nociception after TKA and are not affected by RFA. It is also possible that the routine exposure of the knee in TKA, especially if a synovectomy is performed, may divide the branches of the geniculate nerve(s) upstream from the ablation site, reducing any benefit from prior neurotomy compared to sham.
Study limitations
We see several potential limitations of this study. First, there was significant variation in the time to surgery after study intervention amongst participants. Despite the effects of c-RFA lasting greater than a year (22), we arbitrarily discontinued patients with delays in TKA greater than 12 weeks after study intervention. It is unclear if significant time variation from RFA to surgery has a substantive effect on the effects of RFA in the context of TKA.
We also excluded participants with confounding conditions, including preoperative opioid usage, that are likely to be encountered in clinical practice. We felt that excluding those participants would most likely give an accurate assessment of the effects of ablation procedures.
It is important to emphasize that the effects of RFA neurotomy are considered temporary, and we did not anticipate any longer-term benefits from the use of RFA in TKA. Any longer-term effects of the use of RFA in TKA would require longer follow-up. Given the trends described in the current study, it appears unlikely that there will be any difference with the use of RFA as an adjunct with longer follow-up.
Assessing opioid usage as a primary outcome can be problematic. Patient expectations, family influences, outside provider recommendations, and intrinsic affinity for opioids can all influence opioid usage (24).
Additionally, there has been some scrutiny of the WOMAC instrument and its ability to detect significant clinical differences after knee arthroplasty (25). We acknowledge that there may be better instruments in the future, but at the time of study design, we felt this was the best available validated tool.
Finally, there has been some discussion about the optimal approach to neurolysis for knee pain. Several studies have directly addressed the anatomy of the anterior knee with differing conclusions about appropriate percutaneous targets (10,26,27). It is possible that more sophisticated approaches tailored for presurgical treatment may improve results in the future.
Conclusions
RFA of the genicular nerves prior to TKA did not affect opioid use or cessation, pain, or WOMAC scores following TKA. The results of this clinical trial reinforce the results of a prior similar trial (12). The results also help define the appropriate clinical applications of the current use of geniculate nerve RFA. Therefore, we conclude that current techniques of t-RFA and c-RFA of these specific geniculate nerves are not indicated as routine preoperative interventions to improve short-term surgical recovery after TKA.
Acknowledgments
We thank Mike Ludwig, MD, Greg Bauer, CRNA, and Doran Thomas, CRNA, for performing t-RFA, c-RFA and sham procedures; Ellicia Coyne, MS, for organizing and providing logistical support; Kaisa Kivilaid, MS, for assistance with statistics and data analysis; Jennifer Hunnicutt, PhD, ATC of Hunnicutt Writing and Consulting, LLC for her assistance with editing and writing support.
Funding: This work was supported by Avanos Medical.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-33/rc
Trial Protocol: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-33/tp
Data Sharing Statement: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-33/dss
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-22-33/coif). JRL reports that Avanos Medical, the manufacturer of the c-RFA device, provided necessary funds and training via IORI (The Institute for Orthopedic Research and Innovation), a registered nonprofit organization. Neither Avanos Medical nor its representatives participated materially in study design, collection/interpretation of data, the writing of the manuscript, or the decision to publish the data. None of the authors of this study received personal remuneration for conducting the study. JRL is currently a member of the scientific advisory panel for Avanos Medical. TPL reports his relationship with Stryker is related to teaching hip and knee arthroplasty techniques and the use of robotic technologies. Funds received from Avanos in support of this study were used by the IORI nonprofit research foundation to pay for study-related equipment and research staff salaries. None of the authors of this study were compensated in any way for their involvement. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Providence Hospital Institutional Review Board, Spokane, Washington (IRB# 00000800), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455-60. [Crossref] [PubMed]
- Deshpande BR, Katz JN, Solomon DH, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken) 2016;68:1743-50. [Crossref] [PubMed]
- Kamaruzaman H, Kinghorn P, Oppong R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2017;18:183. [Crossref] [PubMed]
- Missmann M, Grenier JP, Raas C. Modifiable factors influencing length of stay after total knee arthroplasty. Eur J Orthop Surg Traumatol 2022; [Crossref]
- Fuggle N, Curtis E, Shaw S, et al. Safety of Opioids in Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019;36:129-43. [Crossref] [PubMed]
- Trang T, Al-Hasani R, Salvemini D, et al. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J Neurosci 2015;35:13879-88. [Crossref] [PubMed]
- Heckman J, Swiontkowski M. Optimizing Musculoskeletal Pain Management Research in 2020 and Beyond. J Bone Joint Surg Am 2020;102:1-2. [Crossref] [PubMed]
- Ball RD. The science of conventional and water-cooled monopolar lumbar radiofrequency rhizotomy: an electrical engineering point of view. Pain Physician 2014;17:E175-211.
- Kapural L, Deering JP. A technological overview of cooled radiofrequency ablation and its effectiveness in the management of chronic knee pain. Pain Manag 2020;10:133-40. [Crossref] [PubMed]
- Franco CD, Buvanendran A, Petersohn JD, et al. Innervation of the Anterior Capsule of the Human Knee: Implications for Radiofrequency Ablation. Reg Anesth Pain Med 2015;40:363-8. [Crossref] [PubMed]
- Dasa V, Lensing G, Parsons M, et al. Percutaneous freezing of sensory nerves prior to total knee arthroplasty. Knee 2016;23:523-8. [Crossref] [PubMed]
- Walega D, McCormick Z, Manning D, et al. Radiofrequency ablation of genicular nerves prior to total knee replacement has no effect on postoperative pain outcomes: a prospective randomized sham-controlled trial with 6-month follow-up. Reg Anesth Pain Med 2019; [Crossref]
- Chen AF, Khalouf F, Zora K, et al. Cooled Radiofrequency Ablation Compared with a Single Injection of Hyaluronic Acid for Chronic Knee Pain: A Multicenter, Randomized Clinical Trial Demonstrating Greater Efficacy and Equivalent Safety for Cooled Radiofrequency Ablation. J Bone Joint Surg Am 2020;102:1501-10. [Crossref] [PubMed]
- Davis T, Loudermilk E, DePalma M, et al. Twelve-month analgesia and rescue, by cooled radiofrequency ablation treatment of osteoarthritic knee pain: results from a prospective, multicenter, randomized, cross-over trial. Reg Anesth Pain Med 2019;rapm-2018-100051. [Crossref] [PubMed]
- Altman DG. Practical Statistics for Medical Research. Chapman and Hall, 1991.
- Centers for Medicare & Medicaid Services. Prescription Drug Coverage 2018. Available online: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Oral-MME-CFs-vFeb-2018.pdf
- Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28-55. [Crossref] [PubMed]
- Mishra P, Edwards D, Huntoon M, et al. Is preoperative genicular radiofrequency ablation effective for reducing pain following total knee arthroplasty? A pilot randomized clinical trial. Reg Anesth Pain Med 2021;46:752-6. [Crossref] [PubMed]
- Stake S, Agarwal AR, Coombs S, et al. Total Knee Arthroplasty After Genicular Nerve Radiofrequency Ablation: Reduction in Prolonged Opioid Use Without Increased Postsurgical Complications. J Am Acad Orthop Surg Glob Res Rev 2022;6:e22.00125.
- Mihalko WM, Kerkhof AL, Ford MC, et al. Cryoneurolysis before Total Knee Arthroplasty in Patients With Severe Osteoarthritis for Reduction of Postoperative Pain and Opioid Use in a Single-Center Randomized Controlled Trial. J Arthroplasty 2021;36:1590-8. [Crossref] [PubMed]
- Hunter C, Davis T, Loudermilk E, et al. Cooled Radiofrequency Ablation Treatment of the Genicular Nerves in the Treatment of Osteoarthritic Knee Pain: 18- and 24-Month Results. Pain Pract 2020;20:238-46. [Crossref] [PubMed]
- Lyman J, Khalouf F, Zora K, et al. Cooled radiofrequency ablation of genicular nerves provides 24-Month durability in the management of osteoarthritic knee pain: Outcomes from a prospective, multicenter, randomized trial. Pain Pract 2022;22:571-81. [Crossref] [PubMed]
- Nguyen UD, Ayers DC, Li W, et al. Preoperative Pain and Function: Profiles of Patients Selected for Total Knee Arthroplasty. J Arthroplasty 2016;31:2402-2407.e2. [Crossref] [PubMed]
- Onishi E, Kobayashi T, Dexter E, et al. Comparison of Opioid Prescribing Patterns in the United States and Japan: Primary Care Physicians' Attitudes and Perceptions. J Am Board Fam Med 2017;30:248-54. [Crossref] [PubMed]
- Stratford P, Kennedy D, Clarke H. Confounding pain and function: the WOMAC's failure to accurately predict lower extremity function. Arthroplast Today 2018;4:488-92. [Crossref] [PubMed]
- Fonkoué L, Behets C, Kouassi JK, et al. Distribution of sensory nerves supplying the knee joint capsule and implications for genicular blockade and radiofrequency ablation: an anatomical study. Surg Radiol Anat 2019;41:1461-71. [Crossref] [PubMed]
- Tran A, Gonzalez FM. Review of cooled radiofrequency ablation utilization for the treatment of symptomatic advanced knee arthritis and total knee arthroplasty. Skeletal Radiol 2022; [Crossref]
Cite this article as: Lyman JR, Olscamp AJ, Lovell TP, Winegar CD, Wilson AN. Radiofrequency ablation prior to total knee arthroplasty does not improve post-surgical pain or recovery: a double-blinded, multi-center, randomized clinical trial. Ann Joint 2023;8:5.


