
Postoperative infection and bone sarcoma survival: systematic review of the role of infection in bone sarcoma prognosis
Highlight box
Key findings
• Our retrospective study of patients with osteosarcoma or chondrosarcoma found no differences in 5-year survival in the presence of a postoperative infection.
• Our systematic review notes two of six prior studies reported statistically significant findings supporting improved survival for bone sarcoma patients with a postoperative infection.
What is known and what is new?
• Survival rates for osteosarcoma and chondrosarcoma have stagnated in the past 40 years, with survival in metastatic disease as low as 10–20%.
• Immunotherapy may confer a survival benefit in various oncologic illnesses, including in bone sarcoma patients.
What is the implication, and what should change now?
• Immune system modulation due to infection may confer a survival benefit in bone sarcoma. More research must be done to understand the specific circumstances which may elicit a therapeutic response and improved survival, such as the roles played by specific organisms, sarcoma subtype, and immune system activity.
Introduction
Osteosarcoma (OS) and chondrosarcoma (CS) are the most common primary bone sarcomas which occur in pediatric and adult patients, respectively. OS is the most common primary malignant bone tumor and has a worldwide incidence of 3.4 cases per one million people per year (1,2). OS occurs most commonly in younger patients and is the third most common adolescent malignancy, with an incidence of 5.6 cases per one million children under 15 years of age (3). Comparatively, CS is the most common primary bone sarcoma, and has a higher occurrence rate amongst elderly patients (4). Aging populations have contributed to a rising incidence of CS estimated at around 8.8 cases per one million people per year (5). Comparatively, Ewing sarcoma is another common bone sarcoma which is primarily seen in younger patient populations. OS and CS both have known cells of origin arising from bone, whereas Ewing sarcoma’s cell of origin still remains unknown. For the purposes of evaluating primary bone sarcomas known to arise from bone or cartilage cell lines of origin, our subsequent investigation primarily focused on OS and CS. Both OS and CS diagnoses carry significant morbidity and mortality. Ablative surgical resection of the affected bone and surrounding tissues with achievement of negative surgical margins is the foundation of bone sarcoma treatment. Obtaining negative margins on final pathology following surgical resection is a key predictor of local recurrence, metastatic potential, and survival (6,7). The development of multi-agent chemotherapy in the 1970s was a notable advancement which improved OS 5-year survival rates from around approximately 15% to approximately 65% (1). Unfortunately, continued improvements in OS prognoses have stagnated since the 1980s, and few evidence-based treatments have become available with a demonstrated survival benefit in more recent decades (1,8). At present, metastatic OS carries the poorest prognosis, with 5-year survival rates as low as 10% despite the use of multi-agent chemotherapy (9,10). Comparatively, high-grade CS also portends a poor prognosis, with 5-year survival rates ranging from 26–32%, and metastatic disease conferring a 5-year survival rate of 23% (5,11).
Amongst the hallmarks of cancer, evasion of immune destruction is a characteristic which enables tumor cells to persist despite a normal immune system. The capacity of cancer to evade immune surveillance presents a significant challenge in creating effective anti-cancer therapeutics (12,13). In the past, a theory that an enhanced or upregulated immune system could elicit improved reduction of malignant tumors was demonstrated in the work of William B. Coley, considered the “Father of Immunotherapy”. He demonstrated that injecting “Coley’s Toxin”, comprised of various bacteria and bacterial products, into patients with sarcomas resulted in tumor necrosis in some instances (14-16). Implementation of immune modulation via infectious etiologies to treat malignancies faded with the advent of radiation therapy and chemotherapy. While these latter treatments have been widely beneficial in cancer survival, they come with significant tradeoffs (17).
Recent studies have sought to explore if any survival advantage is conferred on sarcoma patients who develop an infection in the postoperative setting (18,19). Bacterial pathogens may accentuate host anti-tumor immune responses in bone sarcoma patients, producing the enhanced response to tumor diminution proposed by Coley’s work (20). Co-culture of Staphylococcus aureus with human macrophages and OS cells have demonstrated increased interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), decreased transforming growth factor beta (TGF-β) cytokine secretion, and increased mRNA expression of TNF-α when compared with macrophages co-cultured with OS and macrophages cultured alone (21). Although this enhanced immune response presumptively occurs in response to an infectious cause, such as Staphylococcus aureus, the activation of the immune system may enhance detection or response to malignant tumor cells. Previous chart reviews of patients with OS and CS have demonstrated this paradoxical relationship (18,19,22). While the literature includes studies which have investigated this phenomenon, the findings of these studies have not been consolidated or reviewed to determine if sufficient evidence exists to support the theory that infection affects survival in sarcoma.
The current study was initiated to (I) determine the effect of postoperative infections in OS and CS patients at a single large academic sarcoma referral center and (II) to review the literature to determine the effect of infection on OS and CS survival. We hypothesize that post-operative infection may augment the immune response such that there is improved immune surveillance of bone sarcoma, and this will be reflected in increased survival observed in bone sarcoma patients who develop infections. The retrospective review portion of this study was completed in accordance with the STROBE reporting checklist, while the systematic review portion was completed in accordance with the PRISMA reporting checklist (available at https://aoj.amegroups.org/article/view/10.21037/aoj-22-41/rc).
Methods
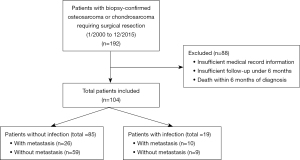
A retrospective case-control study was performed of 192 patients treated for primary OS or CS at a large academic sarcoma referral center in Pittsburgh, PA, USA from January 2000 to December 2015. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Pittsburgh Institutional Review Board (IRB), Institutional Animal Care and Use Committee (IACUC), and Committee for Oversight of Research and Clinical Training Involving Decedents (CORID) (No. PRO10050461) and individual consent for the retrospective analysis portion of the study was waived. Information from each patient was collected from the electronic medical record regarding biopsy results, sarcoma survival status, presence of postoperative wound infection, metastasis, and infectious organism grown on surgical culture. Only patients with (I) a diagnosis of OS or CS made via biopsy and (II) undergoing surgical resection for their malignancy were included in the study. Postoperative wound infections can occur at different time-points, with early infections occurring within 30 days of surgery and late infections developing more than 3 months following surgery (23). In 2017, the Centers for Disease Control (CDC) recommended surveillance of 30 or 90 days following operation for a surgical site infection depending on if any implants were utilized (24,25). In order to capture the broad range of infections that could occur, postoperative wound infections were defined as surgical culture-confirmed infection occurring within 6 months of surgery. After excluding patients with insufficient medical record information, insufficient follow-up under 6 months (e.g., due to loss to follow-up), or death within 6 months of diagnosis, an analysis of 104 patients took place (Figure 1). Survival analysis was performed primarily comparing patients with and without infection for each of the following retrospective cohorts: (I) localized disease without infection, (II) localized disease with infection, (III) metastatic disease without infection, and (IV) metastatic disease with infection. Patients with metastasis were defined based on presence of disease found in any organ distinct from the primary OS or CS site. For surviving patients, follow-up was performed for a minimum of 5 years from the date of diagnosis, with a follow-up endpoint through May 2021. Additional survival analyses were also performed comparing all patients without infection and all patients who developed an infection specifically with culture-positive Staphylococcus aureus growth given the high rate of Staphylococcus aureus seen in orthopedic postoperative infections (26,27). Log rank statistical analysis was utilized to assess differences between groups in terms of overall survival. All calculations were performed using GraphPad Prism Version 9 (GraphPad Software Inc., San Diego, CA, USA) and P values <0.05 were considered significant.
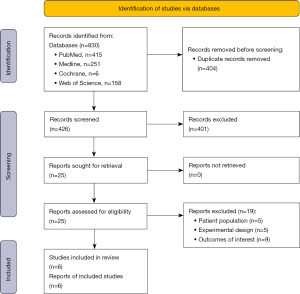
The second portion of the study comprised a systematic review which was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A computerized search of the literature through PubMed, MEDLINE, Web of Science, and Cochrane Library was performed from inception of the databases through September 2022. The search strategy combined terms of “osteosarcoma”, “chondrosarcoma”, and “postoperative infection”, such that “postoperative infection” was searched either in combination with “osteosarcoma” or “chondrosarcoma”, respectively. Study titles, abstracts, and manuscripts were reviewed for their content and published data. Studies were included if they met the following inclusion criteria: (I) participants included in the study had a proven diagnosis of a bone sarcoma and an experimental cohort of subjects had associated infection, including either perioperative or postoperative infection; (II) overall survival outcomes were studied and compared between groups with and without infection, and specific survival rates could be extracted at specific follow-up time points including 5-year survival; (III) the full-text paper was available in English. Exclusion criteria included (I) participants included in the study only had sarcomas (e.g., soft tissue) without a bone etiology; (II) reviews were excluded due to absence of original data, (III) single case reports were excluded given the inherent lack of grouped survival data availability.
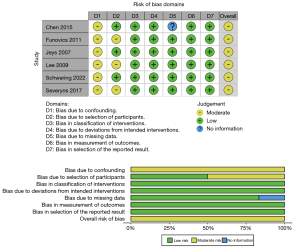
Within the systematic review performed, our initial literature search retrieved 426 publications, 331 articles using “Postoperative Infection + Osteosarcoma” as the terms of the search query and 95 articles using “Postoperative Infection + Chondrosarcoma” as the terms of the search query (Figure 2). Two investigators (MFG, AJF) performed the literature review independently. After consolidation of review, any disagreements on inclusion or exclusion of articles were discussed with manuscript contents reviewed collaboratively. Articles which were excluded on full-text review were primarily due to an inappropriate patient population without primarily bone sarcoma patients (n=5), an experimental design which did not compare patient groups with and without infection (n=5), or did not report the desired outcome of interest of patient survival (n=9). Ultimately, six studies were determined to meet inclusion criteria for the study. Both investigators then collected data independently from each included study, including the year of publication, authors, location, study design, patient cohort, type of infection, 5-year survival rate, and 10-year survival rate. All data points were collected based on availability within each publication. After data collection, data were compared and confirmed between the two study investigators. Upon comparison of data collected, the six studies reviewed had some variability in terms of type of infection reported and time points for survival rates. The decision was made to report type of infection if available, and report survival rates at both 5- and 10-year due to the differences in reporting between publications. All P values reported regarding significance were obtained from log-rank analyses results reported within each individual publication. A study risk of bias assessment was performed utilizing the Cochrane ROBINS-I (Risk of Bias in Non-randomized Studies-of Interventions) assessment tool by a single investigator (MFG) (28). This review was not formally registered and a protocol was not prepared.
Results
For our single institution study, a total of 104 patients were included, with nine patients with non-metastatic disease developing a postoperative wound infection and 10 patients with metastatic disease developing a postoperative wound infection. Overall, patients were 45.1±24.1 years old, were 45.2% male, had 64.4% OS, and had an overall survival of 103.8±63.3 months with 65.4% of surviving patients at end follow-up (Table 1). Overall survival ranked from greatest to lowest in the order of the following groups: (I) non-metastatic disease with infection (88.9%), (II) non-metastatic disease without infection (78.0%), (III) metastatic disease with no infection (42.3%), and (IV) metastatic disease with infection (30.0%), respectively (Figure 3A). Five-year survival was greatest in the group who experienced a postoperative wound infection and did not develop metastasis (100.0%), followed by patients who developed neither infection nor metastasis (89.8%). Five-year survival was lowest in patients with postoperative infection and metastasis (30.0%), followed by patients with metastasis and no postoperative infection (61.5%). The trend for increased survival seen in patients with postoperative infections and no metastatic disease failed to reach statistical significance (P=0.17) in a log-rank statistical analysis. Amongst the 19 patients who developed an infection, six of the infections were polymicrobial in nature. In terms of specific organisms identified, 10 of the infections involved Staphylococcus aureus, three involved coagulase-negative Staphylococcus, and four involved Enterococcus faecalis. Groups with a Staphylococcus aureus infection were compared to the group of patients who did not develop infection and overall survival was greater in the group with Staphylococcus aureus infection (Figure 3B). When comparing patients with and without methicillin-sensitive Staphylococcus aureus (MSSA) or methicillin-resistant Staphylococcus aureus (MRSA) infections, those who developed MSSA/MRSA infections tended to have superior survival; however, this also failed to reach statistical significance (P=0.18).
Table 1
| Group | Age (years) , mean ± standard deviation | Gender (M/F) | Sarcoma type (OS/CS) | Survival (months) , mean ± standard deviation | Surviving patients at end follow-up (%) |
|---|---|---|---|---|---|
| Overall (n=104) | 45.1±24.1 | 45.2% M (n=47), 54.8% F (n=57) | 64.4% OS (n=67), 35.6% CS (n=37) | 103.8±63.3 | 65.4% (n=68) |
| No infection, no metastasis (n=59) | 46.2±26.2 | 33.9% M (n=20), 66.1% F (n=39) | 59.3% OS (n=35), 40.7% CS (n=24) | 117.3±59.4 | 78.0% (n=46) |
| No infection, with metastasis (n=26) | 43.3±20.9 | 50.0% M (n=13), 50.0% F (n=13) | 73.1% OS (n=19), 26.9% CS (n=7) | 82.5±62.0 | 42.3% (n=11) |
| With infection, no metastasis (n=9) | 40.3±26.1 | 77.8% M (n=7), 22.2% F (n=2) | 44.4% OS (n=4), 55.6% CS (n=5) | 118.5±64.9 | 88.9% (n=8) |
| With infection, with metastasis (n=10) | 47.9±19.0 | 70.0% M (n=7), 30.0% F (n=3) | 90.0% OS (n=9), 10.0% CS (n=1) | 66.0±66.3 | 30.0% (n=3) |
M, male; F, female; OS, osteosarcoma; CS, chondrosarcoma.

Among the six articles collected in the systematic review, all six reported cohorts of patients with bone sarcomas with and without infection, as well as associated survival rates (Table 2). Studies which did not meet complete inclusion criteria either had inappropriate patient populations including diagnoses of soft tissue sarcoma or glioblastoma, or reported inappropriate outcomes besides overall or disease-free survival (32,33). Five of the included articles studied the role of infection exclusively in patients with OS, whereas one article evaluated patients with primary bone tumors which included OS and CS patients (29). A total of 1,191 patients were included from all the studies, with 153 patients who developed a postoperative infection. Five of the six studies reported the identification of specific organisms, and Staphylococcus epidermidis/coagulase-negative Staph (60 cases) represented the most common organism, followed by Staphylococcus aureus (31 cases). In total, two of the six studies included found a statistically significant increased survival rate in patients who developed an infection compared to those who did not (18,19). In both respective studies, stark differences were observed with 5-year survival rates of 100% in the infected cohort compared to 54% in the non-infected cohort in the study by Chen et al., and 10-year survival rates of 84.5% in the infected cohort compared to 62.2% in the non-infected cohort in the study by Jeys et al. Four of the six studies included did not find statistically significant differences in either overall survival or disease-specific survival. However, within these studies with statistically insignificant differences, two studies reported “higher” survival rates in the infected cohort, while two reported “lower” survival rates. The ROBINS-I assessment found that all six articles were susceptible to a moderate risk of bias overall, primarily from bias due to confounding and patient selection (Figure 4).
Table 2
| Authors | Year of publication | Study period | Study location | Study design | Patient cohort | Age | Gender, n (%) | Enneking tumor stage (if available), n (%) | Included patients with metastasis at time of surgery | Type of infectious organism (if available) | 5-year survival rate (if available) | 10-year survival rate (if available) | Significance (P value) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. | 2015 | 1991–2012 | Jinan, China (single center) | Retrospective case-control | 125 osteosarcoma patients: 6 infected, 119 non-infected | Infected: 21±11 years; non-infected: 19±8 years | Infected: 3 M (50.0%), 3 F (50.0%); non-infected: 77 M (64.7%), 42 F (35.3%) | 125 stage IIB (100%) | No | 4 Staphylococcus aureus (66.7%), 2 Staphylococcus epidermidis (33.3%) | Infected: 100%; non-infected: 54% | Not reported | 0.01* |
| Funovics et al. | 2011 | 1998–2008 | Vienna, Austria (single center) | Retrospective case-control | 79 osteosarcoma patients: 13 infected, 66 non-infected | Total: 18 years | Total: 42 M (53.2%), 37 F (46.8%) | High grade (unspecified) | No | Not reported | Disease-specific: infected: 59.8%; non-infected: 69.5% | Not reported | 0.600 |
| Jeys et al. | 2007 | 1981–2001 | Birmingham, UK (single center) | Retrospective case-control | 412 osteosarcoma patients: 41 infected, 371 non-infected | Not reported | Not reported | 6 stage I (2%), 25 stage IIA (9%), 221 stage IIB (83%), 16 stage III (6%) | Yes | 23 Staphylococcus epidermidis (52%), 12 Staphylococcus aureus (29%) | Not reported | Infected: 84.5%; non-infected: 62.2% | 0.017* |
| Lee et al. | 2009 | 1990–2003 | Seoul, South Korea (single center) | Retrospective case-control | 93 osteosarcoma patients: 31 infected, 62 non-infected | Infected: ≤15: n=9 (29.0%), >15 to ≤40: n=21 (67.7%), >40: n=1 (3.2%); non-infected: ≤15: n=26 (41.9%), >15 to ≤40: n=35 (56.5%), >40: n=1 (1.6%) | Infected: 24 M (77.4%), 7 F (22.6%); non-infected: 46 M (74.2%), 16 F (25.8%) | 31 stage IIA (33.3%), 62 stage IIB (66.7%) | No | 7 Staphylococcus aureus (22.6%), 5 Staphylococcus epidermidis (16.1%), 2 Enterobacter (6.4%), 1 Pseudomonas aeruginosa (3.2%), 1 group D streptococcus (3.2%), 1 Candida albicans (3.2%) | Infected: 88.9%; non-infected: 82.0% | Infected: 83.3%; non-infected: 82.0% | 0.49 |
| Schwering et al. | 2022 | 1989–2016 | Germany & Austria (multi-center) | Retrospective case-control | 437 osteosarcoma patients: 46 infected, 391 non-infected | Infected: ≤40: n=38 (83%), >40: n=8 (17%); non-infected: ≤40: n=348 (89%), >40: n=43 (11%) | Infected: 29 M (63%), 17 F (37%); non-infected: 240 M (61%), 151 F (39%) | 161 stage I (37%), 248 stage II (57%), 12 stage III (3%), 16 not available (3%) | Yes | 30 Staphylococcus epidermidis (65.2%), 8 Staphylococcus aureus (17.3%) | Disease-specific: infected: 86%; non-infected: 79% | Not reported | 0.286 |
| Severyns et al. | 2017 | 1989–2013 | Nantes, France (single center) | Retrospective case-control | 45 primary bone tumors: 7 osteosarcoma patients: 4 infected, 3 non-infected; 19 chondrosarcoma patients: 9 infected, 10 non-infected; 15 Ewing sarcoma patients: 3 infected, 12 non-infected; 4 other (non-infected) | Infected: 50.2±21.3 years; non-infected: 37.4±19.5 years | Infected: 12 M (75%), 4 F (25%); non-infected: 18 M (62%), 11 F (38%) | Not reported | No | 12 polymicrobial (75%), 3 single organism (18.8%) | Infected: 62.5%; non-infected: 68.9% | Infected: 52.1%; non-infected: 60.1% | 0.924 |
Pertinent 5- and 10-year survival rates included within each article are listed. When pertinent, disease-specific survival rates are denoted if this was the only survival rate published in each article. P values reported were for log-rank analyses performed between infected and non-infected cohorts within each study, with the asterisk (*) denoting a study with statistically significant P values. M, male; F, female.
Discussion
Our study sought to assess the effect of infection on survival in bone sarcoma at our institution and compare these data to the findings in the literature via a systematic review. Our data did not find statistically significant differences between cohorts of patients with or without infection, which contradicted our hypothesis expecting a role for infection in improving survival rates. However, it was notable that the patient cohort with the best survival rates observed in our study was the group of patients with no metastatic disease who developed a postoperative infection. Similarly, we observed that postoperative MSSA/MRSA infection had a better survival rate compared to that of non-infected patients; however, this also did not reach statistical significance. Prior studies have suggested that infection may confer a protective effect in the setting of malignancy, with findings to support this theory as described in our systematic review (18,19). Perhaps with improved power and a larger patient sample size, these findings may have reached statistical significance akin to the studies noted in our review. By contrast, other studies have found no evidence to support infection providing a survival benefit in bone sarcoma, and studies performed on patients with soft tissue sarcomas similarly did not report any significant survival benefit (32). Strengths of the study we performed include surveillance of a broad group of patients with OS and CS, including those with metastases at time of initial evaluation. We also had a long follow-up time and identified inciting organisms in the majority of our patients who developed infection. Limitations of the study include its retrospective nature as well as being underpowered due to the low prevalence of OS and CS, both of which are rare cancers. Our study was also specifically limited by the exclusion of patients who died under 6 months. Within this subset of patients, the presence of infection was undetermined before their death. However, inclusion of these subjects could skew either the infected or non-infected cohort of patients to have presumably shorter survival rates. From our perspective, we believe this exclusion was appropriate as bias towards the non-infected cohort having shorter survival rates would likely exist. At our institution, most patients who died likely did not undergo autopsy, although microbial cultures would have been obtained as part of an infectious work-up in the postmortem examination. In regard to the systematic review we performed, strengths included a strict, well-defined criteria for selection of articles based on patient population, comparison groups, and outcomes. Limitations of the findings of the systematic review were that all of the studies ultimately included were retrospective case-control studies susceptible to a moderate risk of bias.
The theory of improved tumor surveillance elicited by an induced infection or immune response was popularized by the work of William Coley in the 1890s (15). Coley’s foundational work also sought to explore if bone sarcoma survival in the setting of infection could be modified by the type of infectious organism. In his first foray into inducing infection to treat malignancy, he began with live streptococcal organisms with successful shrinkage of tumor at the cost of significant patient morbidity, including two patient deaths (15,34). The second iteration of treatment Coley employed was heat-killed streptococcal organisms combined with Serratia marcescens, which became known as “Coley’s Toxins”, and was used widely from the late 1890s to early 1930s to treat inoperable bone and soft-tissue sarcomas (35). In our study, we specifically explored if Staphylococcus aureus conferred some degree of improved survival in bone sarcoma, with promising but again insignificant results. In other non-sarcomatous neoplasms, Bacillus Calmette-Guerin vaccine, derived from Mycobacterium bovis, is perhaps the best example of a successful immunotherapy used clinically to treat superficial urinary bladder cancer (36). Within the literature for canine OS, a cryopreserved, Listeria-based vaccine has been developed with prolonged survival observed in dogs (37,38). Postoperative infections may confer a protective effect in OS and CS patients by accentuating host anti-tumor immune responses; however, mixed results in the literature suggest that circumstances under which clinically meaningful responses such as rapid tumor regression occur are still unclear. Another predictor of survival in OS which may play a role in quantifying anti-tumor immune response is C-reactive protein (CRP), with findings that lower pre-operative serum CRP is correlated with survival. Patients with a CRP level over 1 mg/dL were found to have a significantly lower disease-specific five-year survival of 36.7% compared to 73.8% in patients with normal values (30). The level of CRP has been noted to cycle in certain malignancies, correlating with intensity of overall inflammation and disease activity, and timing of chemotherapy administration within this cycle may predict treatment response (39). Multiple factors likely predict which patients manifest an anti-tumor response in the setting of postoperative infection, including tumor type, infection type, timing of infection, and more. In patients where an effective response is seen, postoperative infections may confer a protective effect in OS and CS by accentuating host anti-tumor immune responses.
As noted, certain types of malignancies may be specifically susceptible to responding to presence of infection. Even within bone sarcoma, both OS and CS contain over a dozen histological subtypes (40,41). During his research, Coley observed that his treatments preferentially worked on sarcomas, with less efficacy observed in melanomas and carcinomas (42). In fact, the malignancy observed to respond best to Coley’s toxins beyond sarcoma was lymphoma. Prior work on immune modulators such as TNF, interleukin-2 (IL-2), and IFN-α also were most effectively studied in sarcoma models, raising the question whether malignancies of mesodermal origin such as sarcoma are more immunogenic in nature (43). Modern approaches to exploit immune system modulation in order to cure sarcoma has produced clinical trials such as Sarcoma Alliance for Research through Collaboration (SARC) 028, which studied the efficacy of pembrolizumab, an anti-programmed cell death protein 1 (anti-PD1) antibody (44,45). The role of infection in modulating response to malignancy has been explored in tumor types other than sarcoma with mixed findings. In a population of patients with lung carcinoma, a postoperative empyema following surgical resection improved 5-year survival rates, with 50% survival compared to 18% survival observed in a control group (46). The authors theorized that exposure to bacterial antigens activated an immune response which indiscriminately destroyed residual cancer cells while responding to the initial infection. The corollary in bone sarcomas would be osteomyelitis, such that laboratory mouse models of chronic Staphylococcal osteomyelitis in OS have been found to cause significant tumor growth inhibition (47). Perhaps the importance of a local effect is most critical to tumor suppression, much as an empyema in lung carcinoma results in the immune system indiscriminately destroying adjacent cancer cells, so this may be applicable to osteomyelitis in bone sarcoma as well. By contrast, a population of patients with glioblastoma who developed infection after craniotomy was observed not to confer any survival benefit (33). Similarly, analysis of infection in patients who underwent breast cancer resection found no significant survival benefit in the setting of perioperative or post-operative infection (48). Within the head and neck cancer literature, post-operative wound infections were associated with increased recurrence rates and decreased survival against head and neck squamous cell carcinoma (49,50). Simply observing for the presence of postoperative infection and a concomitant survival benefit may be an insufficient way of applying our understanding that infection, under the right circumstances, can facilitate tumor regression. However, the location of the infection, such that there is overlap with the region or type of malignant tissue, may be more critical in predicting whether an anti-tumor response occurs.
In conclusion, our study cohort found that patients with postoperative infection, including MSSA/MRSA infection specifically, did not have statistically significant differences in survival. Further investigation may elucidate the potential role for immunomodulatory therapies in the treatment of OS and CS (20,51). The systematic review component of our study found that, among the few studies performed on infection’s role in bone sarcoma, only a third of studies suggested an association with improved survival. Overall, these findings suggest a role for immune modulation via a pathogen-elicited response to treat malignancies such as bone sarcoma. However, the conditions under which an anti-tumor response can be elicited to improve survival remain unclear. Despite ongoing endeavors to advance surgical, chemotherapeutic, and radiation-based treatments, the stagnant rates of OS and CS survival suggest an alternate approach to treatment may warrant exploration. Future directions to build on this work include improved understanding of the specific factors which may allow patients who develop a postoperative infection to initiate an anti-tumor response, including whether specific organisms, sarcoma histologic subtypes, immune system activity based on biomarkers such as CRP, or location of infection can predict effect on survival. In addition, laboratory animal studies aimed at more directly understanding the potential synergy between anti-pathogen and anti-cancer responses, particularly for specific promising organisms would help build a pathway for developing therapeutics which may improve survival rates in the future.
Conclusions
Our study found that patients who developed postoperative infection following resection of OS or CS did not have statistically significant improvements in survival, including in patients who specifically developed MSSA/MRSA infections. Our systematic review found that 1/3 of studies in the literature have found a role for infection improving survival in bone sarcoma, suggesting an immune response elicited by infection can treat these malignancies. However, many studies failed to find any survival benefit, suggesting an infection-induced anti-tumor immune response may only occur in certain situations. Elucidating the specific circumstances under which an infection-induced therapeutic response can be recreated warrants further investigation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE and PRISMA reporting checklists. Available at https://aoj.amegroups.org/article/view/10.21037/aoj-22-41/rc
Peer Review File: Available at https://aoj.amegroups.org/article/view/10.21037/aoj-22-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.org/article/view/10.21037/aoj-22-41/coif). KRW serves as an unpaid editorial board member of Annals of Joint from March 2018 to February 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Pittsburgh Institutional Review Board, Institutional Animal Care and Use Committee, and Committee for Oversight of Research and Clinical Training Involving Decedents (No. PRO10050461) and individual consent for the retrospective analysis portion of the study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009;125:229-34. [Crossref] [PubMed]
- Misaghi A, Goldin A, Awad M, et al. Osteosarcoma: a comprehensive review. SICOT J 2018;4:12. [Crossref] [PubMed]
- Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med 1999;341:342-52. [Crossref] [PubMed]
- Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv Anat Pathol 2021;28:119-38. [Crossref] [PubMed]
- van Praag Veroniek VM, Rueten-Budde AJ, Ho V, et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg Oncol 2018;27:402-8. [Crossref] [PubMed]
- Biau DJ, Weiss KR, Bhumbra RS, et al. Monitoring the adequacy of surgical margins after resection of bone and soft-tissue sarcoma. Ann Surg Oncol 2013;20:1858-64. [Crossref] [PubMed]
- Salipas A, Dowsey MM, May D, et al. 'Beware the lump in the foot!': predictors of recurrence and survival in bone and soft-tissue sarcomas of the foot and ankle. ANZ J Surg 2014;84:533-8. [Crossref] [PubMed]
- Berner K, Johannesen TB, Berner A, et al. Time-trends on incidence and survival in a nationwide and unselected cohort of patients with skeletal osteosarcoma. Acta Oncol 2015;54:25-33. [Crossref] [PubMed]
- Farfalli GL, Albergo JI, Lobos PA, et al. Osteosarcoma lung metastases. Survival after chemotherapy and surgery. Medicina (B Aires) 2015;75:87-90. [PubMed]
- Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 2019;109:36-50. [Crossref] [PubMed]
- Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long-term follow-up. Acta Orthop 2011;82:749-55. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35:S185-98. [Crossref] [PubMed]
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006;26:154-8. [PubMed]
- Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 1994;64:529-64. [Crossref] [PubMed]
- Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol 2019;10:2965. [Crossref] [PubMed]
- Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg Med Clin North Am 2014;32:167-203. [Crossref] [PubMed]
- Jeys LM, Grimer RJ, Carter SR, et al. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol 2007;14:2887-95. [Crossref] [PubMed]
- Chen YU, Xu SF, Xu M, et al. Postoperative infection and survival in osteosarcoma patients: Reconsideration of immunotherapy for osteosarcoma. Mol Clin Oncol 2015;3:495-500. [Crossref] [PubMed]
- Keung EZ, Burgess M, Salazar R, et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin Cancer Res 2020;26:1258-66. [Crossref] [PubMed]
- Tuohy JL, Somarelli JA, Borst LB, et al. Immune dysregulation and osteosarcoma: Staphylococcus aureus downregulates TGF-β and heightens the inflammatory signature in human and canine macrophages suppressed by osteosarcoma. Vet Comp Oncol 2020;18:64-75. [Crossref] [PubMed]
- Lee JA, Kim MS, Kim DH, et al. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol 2009;16:147-51. [Crossref] [PubMed]
- Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl 1991;73:385-8. [PubMed]
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152:784-91. [Crossref] [PubMed]
- National Healthcare Safety Network, Centers for Disease Control and Prevention. Surgical site infection (SSI) event. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- Li GQ, Guo FF, Ou Y, et al. Epidemiology and outcomes of surgical site infections following orthopedic surgery. Am J Infect Control 2013;41:1268-71. [Crossref] [PubMed]
- Mundhada AS, Tenpe S. A study of organisms causing surgical site infections and their antimicrobial susceptibility in a tertiary care government hospital. Indian J Pathol Microbiol 2015;58:195-200. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Severyns M, Briand S, Waast D, et al. Postoperative infections after limb-sparing surgery for primary bone tumors of the pelvis: Incidence, characterization and functional impact. Surg Oncol 2017;26:171-7. [Crossref] [PubMed]
- Funovics PT, Edelhauser G, Funovics MA, et al. Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop 2011;35:1529-36. [Crossref] [PubMed]
- Schwering C, Niethard M, Gosheger G, et al. Are Postoperative Infections in the First 12 Months after Wide Resection and Megaprosthetic Replacement Associated with the Survival of Osteosarcoma Patients? Results of a Multicenter Study. Cancers (Basel) 2022;14:2682. [Crossref] [PubMed]
- Behnke NK, Alamanda VK, Song Y, et al. Does postoperative infection after soft tissue sarcoma resection affect oncologic outcomes? J Surg Oncol 2014;109:415-20. [Crossref] [PubMed]
- Chen YR, Ugiliweneza B, Burton E, et al. The effect of postoperative infection on survival in patients with glioblastoma. J Neurosurg 2017;127:807-11. [Crossref] [PubMed]
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 1991;3-11. [PubMed]
- Coley WB. The treatment of inoperable sarcoma with the mixed toxins of erysipelas and bacillus prodigiosus. JAMA 1898;XXXI:456. [Crossref]
- Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Anticancer Drugs 2016;27:269-77. [Crossref] [PubMed]
- Mason NJ, Gnanandarajah JS, Engiles JB, et al. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin Cancer Res 2016;22:4380-90. [Crossref] [PubMed]
- Musser ML, Berger EP, Tripp CD, et al. Safety evaluation of the canine osteosarcoma vaccine, live Listeria vector. Vet Comp Oncol 2021;19:92-8. [Crossref] [PubMed]
- Coventry BJ, Ashdown ML, Quinn MA, et al. CRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool? J Transl Med 2009;7:102. [Crossref] [PubMed]
- Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma Overview. Rheumatol Ther 2017;4:25-43. [Crossref] [PubMed]
- Amer KM, Munn M, Congiusta D, et al. Survival and Prognosis of Chondrosarcoma Subtypes: SEER Database Analysis. J Orthop Res 2020;38:311-9. [Crossref] [PubMed]
- Bickels J, Kollender Y, Merinsky O, et al. Coley's toxin: historical perspective. Isr Med Assoc J 2002;4:471-2. [PubMed]
- Starnes CO. Coley's toxins in perspective. Nature 1992;357:11-2. [Crossref] [PubMed]
- Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017;18:1493-501. [Crossref] [PubMed]
- Burgess MA, Crowley J, Reinke DK, et al. SARC 028: A phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with advanced sarcomas. J Clin Oncol 2015;33:abstr TPS10578.
- Ruckdeschel JC, Codish SD, Stranahan A, et al. Postoperative empyema improves survival in lung cancer. Documentation and analysis of a natural experiment. N Engl J Med 1972;287:1013-7. [Crossref] [PubMed]
- Sottnik JL. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother 2010;59:367-78. [Crossref] [PubMed]
- Adwall L, Pantiora E, Hultin H, et al. Association of postoperative infection and oncological outcome after breast cancer surgery. BJS Open 2021;5:zrab052. [Crossref] [PubMed]
- Grandis JR, Snyderman CH, Johnson JT, et al. Postoperative wound infection. A poor prognostic sign for patients with head and neck cancer. Cancer 1992;70:2166-70. [Crossref] [PubMed]
- González-Márquez R, Rodrigo JP, Suárez Nieto C. Prognostic significance of postoperative wound infections after total laryngectomy. Head Neck 2012;34:1023-7. [Crossref] [PubMed]
- Gazouli I, Kyriazoglou A, Kotsantis I, et al. Systematic Review of Recurrent Osteosarcoma Systemic Therapy. Cancers (Basel) 2021;13:1757. [Crossref] [PubMed]
Cite this article as: Dooley SW, Gong MF, Carlson LA, Frear AJ, Mandell JB, Zheng A, Bhogal S, Schoedel KE, Weiss KR. Postoperative infection and bone sarcoma survival: systematic review of the role of infection in bone sarcoma prognosis. Ann Joint 2023;8:22.


