
Usefulness of modular neck adapter in partial hip revision
Introduction
The modularity in total hip arthroplasty (THA) is widely recognized in hip surgery thanks to the advantage of being able to adapt the different acetabular and femoral components geometries to each other (1). However, the incidence of total hip arthroplasty revision (THAr) is increasing due to the high volume of hip prostheses performed worldwide (2,3).
Among the different indications to undergo a new hip surgery after THA, the main ones concern revision of the isolated acetabular component (4), often caused by polyethylene wear and prosthetic dislocation. Stem subsidence, conversions from hemiarthroplasty to THA, and/or ceramic head fracture are other reasons of hip revision (1). Moreover, the recent modular prostheses can lead to different problems like dissociation, breakage, and interfacial fretting corrosion (5-7).
On the other hand, a well-fixed stem component revision usually results difficult to perform and vigorous attempts can carry out to femoral shaft fracture. This event is easier to happen during a second stem re-revision due to the extensively coated prosthesis used previously in the first re-implantation (8). Chung et al. (9) in their study on Paprosky femoral type III defects in 96 femoral revisions achieved stable bony in-growth in 92 cases. Jayakumar et al. (10) demonstrated evidence of bony in‑growth and stable fixation, with no cases of loosening, instability, deep infection, stress shielding, subsidence or osteolysis after 6 years of follow-up. Moon et al. (11) also showed similar results in 35 patients after 77.5 months of follow-up.
In these scenarios of THAr, a modular neck adapter that engages the femoral stem could be useful in order to protect the neck-head junction and restore preoperative biomechanics and soft tissue tension when the femoral or acetabular component should be retained or reoriented. This system allows an arthroplasty revision in case of a well-fixed femoral and acetabular components or where different manufacturing systems are used and an off-label pairing is required (12).
Currently, the only head-neck metal adapters commercialized on the market (13-15) is the Merete BioBall® (Merete Medical, Berlin, Germany) (2,12). These adapters are made of Titanium (TiAl6V4) and are available in several lengths (from −3 to +21 mm identified from S to 5XL) to adapt to the characteristics of different morse tapers (12/14, 14/16, 8/10, 10/12, 11/12, 11/13, V40). Additionally, they have a 7.5° offset and the neck-tapers can be rotated 360 degrees to achieve the best stem neck orientation (Figure 1).
In addition to this, there are available modular heads in different materials (ceramic—Biolox® Delta, CeramTec GmbH, Plochingen, Germany or Biolox® Forte, CeramTec GmbH, Plochingen, Germany, and metals—Vivium® CrNiMnMo-ISO5832-9, Merete Medical GmbH, Berlin, Germany) and sizes (from 28 to 58 mm) (1).
Despite the versatility offered by this system, scientific literature about its clinical utility and related complications is very poor and there is few evidence concerning their real use. Revision hip arthroplasty using a modular head-neck adapter is an infrequent surgical procedure with rare outcomes reported (2). The objective of this narrative review is to assess the BioBall® Merete system in terms of clinical and radiological results when performing THA revision. We present this article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-22/rc).
Methods
The narrative review of the current available literature was conducted in December 2022 through electronic database PubMed, Scopus and Embase. Electronic search was performed independently by two reviewers (Pautasso A and Bardellini G) using the following terms: “Head neck taper” OR “Merete BioBall” AND “revision Total Hip Arthroplasty (MeSH Terms)”. The timeframe was limited between 01/01/2000 and 01/12/2022. Only English-language articles were selected. Published studies that contained data regarding the clinical use of the Merete BioBall® system in hip revision surgery were included, while all the papers concerning modular stem prosthesis and the related neck complications were excluded. The abstract of the selected articles was evaluated. Furthermore, a manual search within the references of the selected articles was performed by the authors (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | December 16th, 2022 |
| Databases and other sources searched | Embase/PubMed/Scopus |
| Search terms used | “Head neck taper” OR “Merete BioBall” AND “revision Total Hip Arthroplasty (MeSH terms)” |
| Timeframe | 2000–2022. Exceptions for references of past classifications [e.g., Brooker Classification (16)] |
| Inclusion criteria | Only English language articles were included |
| Selection process | Pautasso A and Bardellini G conducted independently the research on the electronic database |
| Any additional considerations, if applicable | Abstracts of the selected articles were evaluated |
| Published studies that contained data regarding the clinical use of the Merete BioBall system in hip revision surgery were included | |
| All the papers concerning modular stem prosthesis and the related neck complications were excluded |
Indications
There is no consensus regarding indications for the use of modular neck adapters in THAr.
Hoberg et al. (2), in their study on 95 patients, found out that the 39% of all indications were for recurrent THA dislocations, 38% acetabular component loosening, and 17% acetabular polyethylene liner wear. Forty-four percent of the patients before the implantation of the Merete BioBall® adapter system had one or more revision surgeries.
In the recent systematic review of the Merete BioBall® system described by Novoa et al. (1), which involved 194 patients out of 14 studies included, the primary indication of neck-tapers adapter use was the isolated acetabular cup revision surgery (71.6%), followed by THA instability with recurrent dislocation, stem subsidence and conversion from hemiarthroplasty to THA. In this type of surgery, the authors underline how in some cases the stem’s morse taper presents damage due to the head removal, either bad pre-coupling, breakage of the components and/or poor extraction technique too. This damage could increase the risk of fretting corrosion. When the damage is low, the taper should be protected through metal adapter and the stem could be retained. In case of substantial damage, the neck or the entire stem block should be replaced (13,17). When a metal adapter is used, it must be adjusted to the morse taper characteristics.
Besides protecting the head-neck junction, the system is used to increase the length of the taper, the soft tissue tension and the femoral offset. These features are essential in recurrent dislocation revision surgery (Figures 2,3), as observed by Woelfle et al. (12).
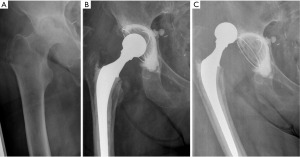
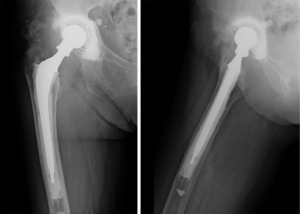
The Merete BioBall® modular head system can help the surgeon to correct femoral stem malpositioning in order to decrease the risk of dislocation. It allows the surgeon to equalize neck length and to correct the stem antetorsion intra-operatively, without the need to revise a stable femoral component, thus avoiding risk of femoral shaft fractures or longer operative times, with simple instrumentation (8).
The most frequent situation where the BioBall system has been useful is in case of a second THAr surgery. The possibility of choosing from a variety of tapers (12/14 and 14/16) is one of the great benefits the adapter offers (2).
Clinical and radiological outcomes
Few studies report clinical and radiological outcomes of the Merete BioBall® modular neck adapter system. Hoberg et al. (2), in their study on 95 patients, did not find any signs of osteolysis, radiolucency or loosening. The use of modular neck adapters did not impact on the formation of periarticular ossifications, with only 16.1% of patients classified as Brooker type I, 4.4% as Brooker type II, and 1.1% as Brooker type III (16). Radiological information about offset restoration and improvement of leg length was demonstrated by Woelfle et al. (12). In their study on 37 patients who performed THA revision with Merete BioBall®, leg length discrepancy passed from 5.8 to 1.2 mm and offset difference improved from 3 to 0 mm. However, clinical results were not as good as the radiological ones, due to high morbidity and old age of the patients involved in the study. Lakstein et al. (18) and De Fine et al. (19) in their studies do not show a significant relationship between leg length or femoral offset restoration and the patient’s functional recovery.
Clinical outcomes were good in the study by Hoberg et al. (2). Patients experienced a Harris Hip Score (HHS) average of 80.9 after surgery with BioBall® System and they were satisfied in 89% of cases. Pain free life was achieved in several cases (86.8%): the mean level of Visual Analogue Scale (VAS) was 1.4. Unfortunately, they did not report any improvement in range of motion (ROM) after surgery.
On the other hand, Woelfle et al. (12) had poorer clinical outcomes: the HHS average post-surgery was 54.0 in their series on 37 patients. These patients were older and obtained a low clinical outcome with 20% rate of second revisions. These factors show the restricted and limited indications for the use of modular neck adapters like the Merete BioBall® system.
Dabis et al. (20), in a study on 32 patients treated with the Merete BioBall® modular neck adapters, had a very good clinical outcome with 6.3% post-operatory recurrent dislocation rate and a significant improvement in the Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire, confirming the functionality and effectiveness of the system.
Related complications and implants survival
The modularity augmentation in the implants could lead to a well-known phenomenon like dissociation, breakage and interfacial fretting corrosion. In this related case, the modular system could potentially conduct to an adapter-neck junction failure or to an earlier corrosion of the materials. The risk of corrosion has been investigated by Kretzer et al. (21): they conducted an in vitro study in which they compared four different modular stem prosthesis (Eco-Modular®, Endoplant, Marl, Germany; Varicon®, Falcon Medical, Mödling, Austria; Metha®, Aesculap, Tuttlingen, Germany; SPS-Modular®, Symbios, Yverdon, Switzerland), and the universal modular neck adapter (BioBall®, Merete, Berlin, Germany), finding out that the metal ions released from the implants interface was very low. They also proved that fretting corrosion was minimal when applying forces of normal gait. Nevertheless, fretting corrosion remains a big deal in modular hip endoprosthetics: various studies report up to 34.5% of corrosion when scanning with electron microscopy the components explanted during revisions (6,22,23). In two recent reviews, during BioBall® revision surgeries, the authors did not find any clinical signs of corrosion or fretting (1,2).
The mechanism of modular components failure happens in two modalities: fatigue fractures of the neck adapter caused by surface cracks (24) and third body wear that leads to periprosthetic osteolysis (25).
In literature, Lizano-Díez et al. (26) reported one case of stem neck fracture in a patient with a BioBall® 4XL adapter, which happened two years after an isolated acetabular cup revision. This is the only known case of this complication and it could be due to the elevated force created by such a long neck adapter on the stem neck.
Another possible complication cited in literature is the ceramic head fracture when using the BioBall® adapters. Jack et al. (27) and Habermann et al. (28) had a ceramic head fracture using a 32 mm and a 28 mm alumina ceramic head (Biolox® Forte®), respectively. It is important to note that no cases of ceramic head fracture have ever been reported when using Biolox® Delta® ceramic heads (8,17). As a matter of fact, the Merete BioBall® adapters are coupled only with fourth generation ceramic heads: Biolox® Delta®. In the projecting phase, the modular neck adapters were subjected to the tests of breaking strength (ISO 7206-4; ISO 7206-6; ISO 7206-8; ASTM F2068; ISO 7206-10 and ASTM C1465-08) with good results.
Another possible issue may be the disassembly of the system, especially when using the longest adapters in addition to the offset configuration (29). To date, there are not any known cases of this phenomenon reported in literature: other reviews such as the one by Novoa et al. (1) and the one by Hoberg et al. (2) confirm this thesis. Hoberg et al. (2) reported in their study a 92.8% [95% confidence interval (CI): 84–95%] implant survival at 8.17 years: this confirms the good survival of the adapter. Nevertheless, more studies are needed to confirm the long-term outcome presented.
In addition to this, it is important to know that the BioBall® adapters cannot be coupled with femoral heads under 28 mm of diameter. As such, in case of a revision of a dual mobility cup of small diameter (30), the system has not to be taken into account.
Actually, the most important problem during the use of BioBall® adapters is recurrent hip dislocation after its use. The re-dislocation rates reported in literature vary from 5.2% (2) to 15% (12). The adapter system cannot always compensate big version defects of the acetabular cup and the stem. In these cases, a revision of the components is necessary to obtain a stable hip arthroplasty.
Conclusions
The modular neck adapter system seems to be a good surgical procedure for recurrent dislocation of THA with a well-fixed stem but not positioned correctly or during an isolated acetabular cup revision, which is actually the main indication of implant use. The literature is very poor on this matter and the long-term outcomes have yet to be proven in more clinical trials.
However, the taper adapter system allows different length and offset neck changes to reach a stable THA, it permits the surgeon to perform a quick revision without removing the existing components, and finally, the great flexibility and precision of the system results helpful in cases of unexpected surgical situations like unstable hip prosthesis. Some possible complications related to the implant design were reported but as isolated cases. The neck adapter failure or corrosion phenomena have not been reported to date in literature.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Solarino and Giuseppe Marongiu) for the series “Modular Implants for Revision Arthroplasty in Orthopedics” published in Annals of Joint. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-22/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-22/coif). The series “Modular Implants for Revision Arthroplasty in Orthopedics” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Novoa CD, Citak M, Zahar A, et al. The Merete BioBall system in hip revision surgery: A systematic review. Orthop Traumatol Surg Res 2018;104:1171-8. [Crossref] [PubMed]
- Hoberg M, Konrads C, Huber S, et al. Outcome of a modular head-neck adapter system in revision hip arthroplasty. Arch Orthop Trauma Surg 2015;135:1469-74. [Crossref] [PubMed]
- Kurtz S, Mowat F, Ong K, et al. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am 2005;87:1487-97. [PubMed]
- He C, Feng JM, Yang QM, et al. Results of selective hip arthroplasty revision in isolated acetabular failure. J Surg Res 2010;164:228-33. [Crossref] [PubMed]
- Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty 2009;24:1019-23. [Crossref] [PubMed]
- Goldberg JR, Gilbert JL, Jacobs JJ, et al. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res 2002;149-61. [Crossref] [PubMed]
- Goldberg JR, Gilbert JL. In vitro corrosion testing of modular hip tapers. J Biomed Mater Res B Appl Biomater 2003;64:78-93. [Crossref] [PubMed]
- Vaishya R, Sharma M, Chaudhary RR. Bioball universal modular neck adapter as a salvage for failed revision total hip arthroplasty. Indian J Orthop 2013;47:519-22. [Crossref] [PubMed]
- Chung LH, Wu PK, Chen CF, et al. Extensively porous-coated stems for femoral revision: reliable choice for stem revision in Paprosky femoral type III defects. Orthopedics 2012;35:e1017-21. [Crossref] [PubMed]
- Jayakumar P, Malik AK, Islam SU, et al. Revision hip arthroplasty using an extensively porous coated stem: medium term results. Hip Int 2011;21:129-35. [Crossref] [PubMed]
- Moon KH, Kang JS, Lee SH, et al. Revision total hip arthroplasty using an extensively porous coated femoral stem. Clin Orthop Surg 2009;1:105-9. [Crossref] [PubMed]
- Woelfle JV, Fraitzl CR, Reichel H, et al. Significantly reduced leg length discrepancy and increased femoral offset by application of a head-neck adapter in revision total hip arthroplasty. J Arthroplasty 2014;29:1301-7. [Crossref] [PubMed]
- Güttler T. Experience with BIOLOX®OPTION revision heads. Benazzo F, Falez F, Dietrich M. editors. Bioceramics and alternative bearings in joint arthroplasty. Steinkopff Verlag Darmstadt; 2006:149-54.
- Windhagen H, Chincisan A, Choi AF, et al. Soft-Tissue Balance in Short and Straight Stem Total Hip Arthroplasty. Orthopedics 2015;38:S14-S20. [Crossref] [PubMed]
- Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am 2007;89:1832-42. [PubMed]
- Brooker AF, Bowerman JW, Robinson RA, et al. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am 1973;55:1629-32. [Crossref] [PubMed]
- Helwig P, Konstantinidis L, Hirschmüller A, et al. Modular sleeves with ceramic heads in isolated acetabular cup revision in younger patients-laboratory and experimental analysis of suitability and clinical outcomes. Int Orthop 2013;37:15-9. [Crossref] [PubMed]
- Lakstein D, Atoun E, Wissotzky O, et al. Does restoration of leg length and femoral offset play a role in functional outcome one year after hip hemiarthroplasty? Injury 2017;48:1589-93. [Crossref] [PubMed]
- De Fine M, Romagnoli M, Toscano A, et al. Is there a role for femoral offset restoration during total hip arthroplasty? A systematic review. Orthop Traumatol Surg Res 2017;103:349-55. [Crossref] [PubMed]
- Dabis J, Hutt JR, Ward D, et al. Clinical outcomes and dislocation rates after hip reconstruction using the Bioball system. Hip Int 2020;30:609-16. [Crossref] [PubMed]
- Kretzer JP, Jakubowitz E, Krachler M, et al. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop 2009;33:1531-6. [Crossref] [PubMed]
- Fraitzl CR, Moya LE, Castellani L, et al. Corrosion at the stem-sleeve interface of a modular titanium alloy femoral component as a reason for impaired disengagement. J Arthroplasty 2011;26:113-9, 119.e1.
- Hernigou P, Queinnec S, Flouzat Lachaniette CH. One hundred and fifty years of history of the Morse taper: from Stephen A. Morse in 1864 to complications related to modularity in hip arthroplasty. Int Orthop 2013;37:2081-8. [Crossref] [PubMed]
- Grupp TM, Weik T, Bloemer W, et al. Modular titanium alloy neck adapter failures in hip replacement--failure mode analysis and influence of implant material. BMC Musculoskelet Disord 2010;11:3. [Crossref] [PubMed]
- Urban RM, Jacobs JJ, Gilbert JL, et al. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am 1994;76:1345-59. [Crossref] [PubMed]
- Lizano-Díez X, Alentorn-Geli E, León-García A, et al. Fracture of the femoral component after a lightning strike injury: A case report. Acta Orthop Traumatol Turc 2017;51:84-7. [Crossref] [PubMed]
- Jack CM, Molloy DO, Walter WL, et al. The use of ceramic-on-ceramic bearings in isolated revision of the acetabular component. Bone Joint J 2013;95-B:333-8. [Crossref] [PubMed]
- Habermann B, Ewald W, Rauschmann M, et al. Fracture of ceramic heads in total hip replacement. Arch Orthop Trauma Surg 2006;126:464-70. [Crossref] [PubMed]
- D'Angelo F, Zagra L, Moretti B, et al. Retrospective multi-centre study on head adapters in partial revision hip arthroplasty. Hip Int 2020;30:72-6. [Crossref] [PubMed]
- Neri T, Boyer B, Batailler C, et al. Dual mobility cups for total hip arthroplasty: tips and tricks. SICOT J 2020;6:17. [Crossref] [PubMed]
Cite this article as: Pautasso A, Bardellini G, Stissi P, D’Angelo F. Usefulness of modular neck adapter in partial hip revision. Ann Joint 2023;8:35.



