
Bipolar bone loss and distance to dislocation
Introduction
As the glenohumeral joint is the most commonly dislocated joint in the human body, anterior shoulder instability is a familiar orthopedic problem, particularly affecting young, hyper-lax contact athletes and military personnel at a rate as high as 3% per year (1-4). Research has shown that a single dislocation decreases the force needed for subsequent dislocations and increases the risk of future instability episodes (5,6). Repeated dislocations often result in glenoid- and humeral-sided bone loss in the forms of bony Bankart and Hill-Sachs lesions, respectively. This bone loss perpetuates further instability and potential failure after initial stabilization (2,5,7). Studies have shown that glenoid- and humeral-sided bone loss may be present in up to 73–93% of individuals with recurrent instability. As such, it must be addressed appropriately, as the amount of bone loss drives surgical decision making and influences outcomes (8-11). Methods to describe and measure bone loss have changed over time. Originally, glenoid and humeral bone loss were viewed separately. However, the concepts of bipolar bone loss, the glenoid track (GT), and “on/off-track” lesions arose, highlighting the interplay between the two entities in contributing to recurrent instability (12-15). More recently, further attention has been given to “on-track” lesions. The new concept of “distance to dislocation” (DTD) has gained popularity and suggests that not all “on-track” lesions should be treated in the same manner (16).
Whether bone loss is glenoid-sided, humeral-sided, or bipolar, the assessment of bone loss plays a crucial role in the treatment of anterior shoulder instability. Meticulous review of the current available literature was assessed, and the purpose of this article is twofold: (I) describe glenoid, humeral, and bipolar bone loss in the setting of anterior shoulder instability; and (II) elaborate on the new concept of “DTD” and its use in guidance of management.
Bone loss in shoulder instability
Glenoid bone loss
Successful treatment of both primary and recurrent anterior shoulder instability requires careful consideration of glenoid bone loss, as failure to address underlying bony deficiency confers an increased risk of recurrent instability (17). Treatment algorithms for glenoid bone loss focus on defining “critical” versus “subcritical” thresholds, although the precise definitions of each remain debated (18). While critical anterior bone loss is typically defined as 20–25% of the glenoid diameter (19), studies have found increased failure rates with capsulolabral repair in patients with as little as 13.5% glenoid bone loss (18). Thus, thorough preoperative planning with accurate image-based measurements is critical in the management of patients with glenohumeral instability and underlying glenoid bone loss.
Assessment of glenoid bone loss
Following a thorough history and physical examination, imaging evaluation of the patient with suspected glenoid bone loss should begin with dedicated shoulder radiographs. A standard series includes anteroposterior (AP), true AP or Grashey, scapular Y, and axillary lateral views. AP views may demonstrate coronal plane translation of the humeral head as well as loss of the sclerotic margin of the anterior glenoid, while the axillary view can reveal axial subluxation and anterior glenoid deficiency (20). Glenoid bone loss is best elicited radiographically with a West Point view, a modification of the axillary lateral (21). However, as the sensitivity of such projections is limited, advanced imaging is recommended for further evaluation and preoperative planning (22).
Computed tomography (CT) scans enable more complete evaluation of osseous anatomy in acute injury and chronic bone loss. Characterization of anterior glenoid rim fractures (“bony Bankart” lesions) is important, as attritional bone loss may develop if such injuries are not appropriately addressed, increasing the size of the glenoid defect (23). In chronic bone loss, CT scan is indicated to determine the size and location of osseous deficiency. Multiple measurement techniques exist, including linear and area-based measurements.
Most area-based, as well as some linear-based, techniques employ a best-fit circle to measure glenoid bone loss. Two types of glenoid best-fit circle have been described: the “inner circle” (24) and the “outer circle” (25). The inner circle is defined as a circle fitting the inferior glenoid face, while the outer circle is defined as a circle connecting the most superior and inferior portions of the glenoid. While the inner, or inferior, circle is more commonly used, in the setting of glenoid bone loss the outer circle method may be technically easier and more reproducible. A recent study noted a high correlation between the inner and outer circle measurements, with a ratio of 0.74 (26). Although future studies are needed to confirm the validity of this ratio, it may serve as a useful technique to mitigate variations in measurement attributable to the presence of bone loss.
Popular area-based techniques for measuring glenoid bone loss, such as the “Pico” (27) and Sugaya (24) methods, typically derive from inner circle measurements. In the Pico method described by Baudi et al., a sagittal two-dimensional (2D) CT image is selected, providing an en face view of the glenoid. A circle of best fit is placed along the posteroinferior curvature of the contralateral (uninjured) glenoid, and its area measured. The circle is then superimposed on the injured side. The area of bone loss anteriorly is measured and digitally subtracted from the overall area, giving a percentage area of deficiency. In the presence of a bony Bankart lesion, the best fit circle is drawn on the injured side, while the area of the bony fragment is similarly measured and subtracted to determine the degree of deficiency (27). The Sugaya method is comparable yet uses a sagittal three-dimensional (3D) CT image. The circle of best fit is drawn based on the inferior portion of the glenoid from the 3 o’clock to 9 o’clock positions, while the size of the osseous fragment or defect is calculated using CT-based software. The area of the defect is then divided by the area of the best fit circle, yielding the percentage of bone loss (24).
Numerous linear-based measurements have also been described (28-31). Griffith et al. described the “Griffith Index”, which involves drawing a line from the supraglenoid tubercle to the infraglenoid tubercle on the uninjured side (line B), followed by a perpendicular line spanning the widest portion of the glenoid (line A). This is repeated on the injured side, and the ratio of B/A is compared between the two. A ratio of 0.7 is considered normal, while a smaller ratio is noted on the injured side (28). However, while this technique enables easy and reliable identification of anterior glenoid bone loss, its utility in guiding prognosis and treatment is limited (32). A more recent and commonly performed linear-based measurement was described by Sugaya (31). A circle of best fit is drawn on the injured glenoid, and the diameter measured. The maximum width of the anterior defect is then measured and divided by the diameter to yield a percentage of bone loss (31). Finally, the “AP distance to the bare area” method uses the center of an inferiorly based circle of best fit as its primary landmark. The horizontal distances from the center of the glenoid to the anterior edge (A) and the posterior edge (B) are measured, and bone loss is calculated according to the formula: (B − A)/2B × 100% (30). While linear-based measurements are advantageous in their convenience and reproducibility, recent studies have demonstrated that such measurements may overestimate glenoid bone loss by as much as 7% compared to area-based and arthroscopic measurements (33,34). Furthermore, maximum error is present when theorized bone loss is 20%, a commonly accepted threshold for critical bone loss, with subsequent implications for treatment (33). For these reasons, the authors prefer the use of area-based measurements such as the Pico method when calculating anterior glenoid bone loss.
When comparing 2D versus 3D CT, studies indicate that 3D CT provides more reproducible measurements than 2D with the use of a standardized en face view (32,35). Furthermore, 2D CT is highly dependent on formatting of cuts in the plane of the body versus the scapula, which may lead to under- or overestimation of glenoid bone loss based on the level of the cut (36). Thus, when available, 3D CT is the preferred modality for calculating glenoid bone loss. The relationship between CT and magnetic resonance imaging (MRI) is less well-defined. CT is widely considered the “gold standard” imaging modality due to superior bony resolution and availability (32), yet recent studies suggest that 2D and 3D MRI is equivalent to 3D CT in evaluating bone loss (37-40). Thus, when determining the optimal study, the risk of ionizing radiation characteristic of CT must be weighed against the cost and limited availability of MRI (2D or 3D). It is also important to note that MRI is useful in evaluating for soft tissue or chondral injuries that may occur concomitantly with glenohumeral dislocation, including rotator cuff tears, humeral avulsions of the glenohumeral ligament (HAGL) lesions, or glenoid articular cartilage defects (20).
Defining glenoid bone loss
While surgery is often indicated in patients with glenohumeral instability and associated bone loss, treatment is guided by the degree and pattern of bony deficiency. However, determining clinically significant thresholds of glenoid bone loss remains difficult due to a lack of consensus throughout the literature. In general, treatment algorithms for glenoid bone loss focus on defining “critical” versus “subcritical” limits of bony deficiency, both of which will be discussed.
Critical bone loss
One of the earliest descriptions of “critical” bone loss was by Itoi et al. in 2000 (25). In a cadaveric study, the authors demonstrated that an anterior osseous defect with a width measuring at least 21% of the glenoid length was associated with instability and limitations in range of motion following isolated Bankart repair (25). This work was expanded upon by Lo et al., who found the presence of 25–27% bone loss to produce an inverted pear glenoid, and correlated this morphology with the need for a bony augmentation procedure (19). Since that time, glenoid bone loss >20–25% has consistently been defined as critical, as numerous studies have shown high rates of recurrent instability following isolated capsulolabral repair in such patients (41,42). A recent scoping review found that 60.5% of included studies used this threshold as the determining factor when deciding to perform a soft tissue or bony augmentation procedure such as a Latarjet or bone block allograft (43). However, the authors also highlighted the emerging significance of subcritical bone loss, with increased failure rates following soft-tissue stabilization in patients with anterior glenoid bone loss of 15% or less (18).
Subcritical bone loss
Studies within the last decade have challenged the notion that patients with anterior glenoid bone loss measuring less than 20% may be successfully treated with soft tissue stabilization. In 2014, Shaha et al. reported increased rates of recurrent instability following primary arthroscopic repair in patients with >17% bone loss (44); the following year, the same group demonstrated inferior patient-reported outcomes following capsulolabral repair in patients with bone loss above 13.5% (18). Since that time, numerous studies have reported similar findings, with some calling for the redefinition of critical bone loss as anywhere from 15% (45) to 17% (46). Even more recently, a 2018 study reported the glenoid bone loss as low as >10% may be a threshold for bony augmentation procedures (47). In a 2022 scoping review of the assessment and management of glenohumeral bone loss, Gouveia et al. found that 34% of included studies used a threshold of 15% or less when deciding the appropriate amount of bone loss to perform an isolated soft tissue procedure. Notably, the authors also reported a trend based on the year of publication, with more recent studies reporting lower thresholds for the consideration of bony augmentation procedures (43).
Authors’ preferred treatment
Despite recent literature that directs the treatment of recurrent glenohumeral instability based on glenoid bone loss, it is important to note that this factor cannot be considered in isolation. Numerous other morphologic and patient-specific factors contribute to the risk of recurrent instability and should be weighed when deciding on the optimal treatment for a specific patient. Such factors include age, participation in competitive, contact, or overhead sports, the presence of hyperlaxity (48), and concomitant Hill-Sachs lesions or bipolar bone loss (49). However, stratification of degrees of glenoid bone loss can be helpful in determining a basic algorithm to help guide treatment.
After obtaining appropriate preoperative imaging using 3D CT, the authors prefer to divide patients into four categories based on the following degrees of glenoid bone loss: >40%, 20–40%, 13.5–<20%, <13.5%. Patients with >40% glenoid bone loss often require a larger graft and are indicated for free bone block augmentation using either distal tibia allograft (DTA) or autograft or iliac crest bone graft (ICBG) allograft or autograft. In patients with 20–40% glenoid bone loss, the authors perform Latarjet due to the combined sling effect of the conjoint tendon and bony reconstruction of the coracoid (50).
Patients with <20% glenoid bone loss are managed differently according to risk factors including age and status as a contact athlete, as well as morphologic features such as the presence of bipolar bone loss. In such patients, treatment is guided by the accurate assessment of humeral-sided bone loss, as well as the interplay between the GT and any existing Hill-Sachs lesion.
Humeral bone loss
While evaluating glenoid bone loss is a critical component of determining the extent of injury after dislocation, it is also key to evaluate humeral-sided bone loss. The Hill-Sachs lesion is an important independent risk factor of anterior shoulder instability and is often indicative of high energy dislocation events (51). Hill-Sachs lesions are present in an estimated 40–90% of cases of anterior shoulder dislocation events, and as high as 100% of cases of recurrent anterior shoulder instability (51).
Like glenoid-sided defects, the location and size of humeral bone loss is an important component of the algorithm for treatment of shoulder instability. However, determining the extent of humeral sided defects is limited by a lack of universal and consistent preoperative measurement criteria (52).
Measuring humeral bone loss
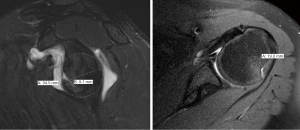
A variety of methods for measuring humeral bone loss exist. While radiography has historically been a modality for measuring the extent of humeral defects (53,54), advanced imaging modalities (i.e., CT and MRI) have been widely recognized as more accurate and reliable (22). Regardless of the method, the goal is to determine both the dimensions of the Hill-Sachs lesion as well as the Hill-Sachs interval (HSI), which measures the distance from the medial edge of the Hill-Sachs lesion to the articular rotator cuff insertion (55).
In 2011, Cho et al. used CT with 3D reconstruction to predict which Hill-Sachs lesions had the highest rate of recurrent anterior shoulder instability. The Hill-Sachs lesion was found by identifying the cut with the greatest width in both the axial and coronal planes, which provided both the length and the depth of the lesion (56). In 2014, Ozaki et al. used 3D CT and found that CT was able to detect almost two-thirds of Hill-Sachs lesions; however, while CT was appropriate for osseous lesions, it did not reliably detect cartilaginous lesions (57). Meanwhile, the use of MRI, while more recent, has proven useful in the characterization of humeral bone loss in patients with glenohumeral instability. In an early feasibility study, Gyftopoulos et al. evaluated both glenoid and humeral bone loss using MRI, resulting in an 84.2% accuracy rate (58). Additionally, magnetic resonance arthrography (MRA) has been especially useful in the determination of not only Hill-Sachs length and width, but also the volume of the defect, resulting in a sensitivity and specificity ranging from 69% to 100% and 0% to 100%, respectively (59). While there are many methods and modalities of characterizing humeral bone loss, no consensus exists on the best, easiest, and most reliable method. Additionally, because of the low interobserver reliability and high variability in measurements of Hill-Sachs lesions seen with various methods of measurement (52,60,61), developing treatment strategies solely based on the Hill-Sachs lesion is not recommended.
Authors’ preferred method of measurement
The authors prefer the use of MRA for the characterization of Hill-Sachs lesions. Patients with anterior shoulder instability will routinely receive magnetic resonance (MR)-arthrograms as a diagnostic and preoperative planning tool. On MRA, we prefer the use of the T1-weighted sequence on axial imaging to determine the width and depth of the Hill-Sachs lesion. Each patient’s Hill-Sachs lesion is then characterized as either absent, mild, moderate, and severe, based on the Rowe classification (62). However, as the Hill-Sachs lesion is just one element of the treatment algorithm, it is essential to consider both glenoid- and humeral-sided defects to understand the severity of injury and how to best address glenohumeral instability after traumatic anterior shoulder dislocations.
Assessing bipolar bone loss
It is well described that the interplay between humeral- and glenoid-sided bone loss contributes to failure of soft tissue shoulder stabilization. In 2000, Burkhart and De Beer first recognized the importance of bipolar bone loss as they found that inverted-pear glenoid morphology in combination with engaging Hill-Sachs lesions were risk factors for recurrence after isolated arthroscopic Bankart repair (13). In 2007, Yamamoto et al. developed the GT concept, determining that a Hill-Sachs lesion had risk of engagement and dislocation if it extended over the medial margin of the GT (12). The GT can be calculated with the formula “0.83D − d”, where “D” is the diameter of the glenoid fit to a perfect circle on a sagittal CT or MRI image (mm) and “d” is the amount of glenoid bone loss, measured from the edge of the glenoid to the rim of the perfect circle (mm) (12,14,63). The HSI should be measured on axial CT or MRI imaging and represents the width of the Hill-Sachs lesion (mm) plus the width of the intact bone bridge (mm) between the rotator cuff attachment and the lateral margin of the Hill-Sachs lesion (Figure 1) (12,14,64). Di Giacomo et al. classified Hill-Sachs lesions as “on-” or “off-track”, and subsequent analyses have validated this method, showing that the risk of recurrent instability is higher when “off-track” lesions (HSI > GT) are treated nonoperatively or with Bankart repair in isolation (14,65,66).
Rather than relying solely on glenoid- or humeral-sided measurements, addressing bone loss as a bipolar concept continues to gain popularity and aids in better understanding the dynamic nature of the unstable shoulder. MRI, 3D CT scans, and standard CT scans have all proven acceptable imaging modalities for assessing the nature of a Hill Sachs lesions as “on-” or “off-track” (14,58,63). A 2022 scoping review published by Gouveia et al. compiled recent findings related to methods for assessment of bone loss in anterior shoulder instability. They included 113 studies in their review. Of these studies, 23.9% (27/113) utilized the GT concept in addressing bipolar bone loss by 3D CT (13 studies), standard CT (7 studies), or MRI imaging (5 studies). Interestingly, the authors also found that the use of the GT concept grew in popularity over the search period. In the earlier half of the search period [2017–2019], the GT was reported in <15% of studies (7 of 47), while in the latter half [2020–2022], 30% of studies (20 of 66) utilized the concept to examine the bipolar nature of bone loss (43). While the GT concept has been monumental in assessing and quantifying bipolar bone loss, current research has further classified “on-track” lesions, as these lesions should not all be viewed equally.
“DTD”
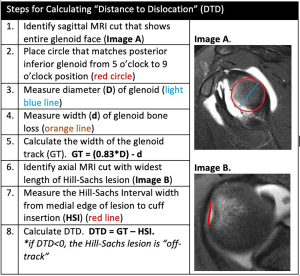
The presence of “off-track” Hill-Sachs lesions greatly increases the risk for recurrent instability, even after isolated Bankart repair (65). However, not all “on-track” lesions should be viewed in the same manner (64). In 2021, Li et al. introduced the new concept of “DTD” in the setting of “on-track” Hill-Sachs lesions. The authors defined DTD as the distance from the medial edge of the Hill-Sachs lesion to the medial edge of the GT (calculated as DTD = GT − HSI; Figure 2) and concluded “on-track” lesions with DTD less than 8 mm (“near-track” lesions) increased the rate of failure after arthroscopic Bankart repair (Figure 3) (16). Research on “near-track” lesions and DTD continues to evolve. In 2022, Barrow et al. reported on 188 individuals with “on-track” lesions undergoing arthroscopic Bankart repair with minimum 2-year follow-up. Amongst other predictors of failure, the authors concluded that as DTD approached 0 mm (“off-track” threshold), the risk of recurrent dislocation after arthroscopic Bankart repair significantly increased. Furthermore, below a DTD threshold of 10 mm, the risk of failure increased exponentially across the study population. In collision sports athletes, recurrent dislocation risk remained elevated at higher DTD values (24 mm) than for noncollision athletes (49).
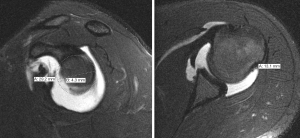
Currently, research involving the DTD concept continues to grow. Studies examining rates of failure after arthroscopic labral repair compared to arthroscopic labral repair plus remplissage in patients with “near-track” lesions are underway. Furthermore, research currently examining the effects of hyperlaxity, Hill-Sachs’s lesion location, and DTD in patients with subcritical bone loss and those who’ve experienced recurrent instability after Latarjet procedure is underway.
Authors’ preferred assessment and treatment of bipolar bone loss
In cases of suspected bone loss based on history, examination, and radiographs, advanced imaging is obtained including both CT and MRI. These studies are performed to evaluate the magnitude and location of osseous deficiency, anchors from prior capsulolabral repair, and the integrity of surrounding soft tissue structures.
In the presence of an off-track Hill-Sachs lesion, a remplissage is performed. For contact athletes, consideration is also given to performing an open Bankart repair or a Latarjet, as studies have demonstrated decreased rates of recurrence with the use of these procedures in athletes compared to arthroscopic repair (67,68). For on-track lesions, as the DTD approaches 0 mm, the risk of recurrent dislocation increases exponentially, particularly in contact athletes (34). Thus, in contact athletes demonstrating “near-track” lesions with subcritical bone loss and a DTD ≤10 mm, consideration is given to combined Bankart repair and remplissage, or Latarjet (16,49).
Conclusions
Bone loss in shoulder instability drives treatment algorithms and patient outcomes. Humeral- and glenoid-sided bone loss must be measured and addressed in the unstable shoulder to promote the most successful outcomes. The new concept of DTD introduces an additional dimension to the GT concept and has been proven as a predictor of failure after arthroscopic stabilization. As the body of literature continues to grow on DTD, the concept will provide necessary information for surgeons, offering support for specific management options following anterior shoulder instability.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Bone Loss in Shoulder Instability and Shoulder Arthroplasty”. The article has undergone external peer review.
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-17/coif). The series “Bone Loss in Shoulder Instability and Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. J.D.H. served as an unpaid Guest Editor of the series. A.L. served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from December 2022 to November 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohtadi NG, Chan DS, Hollinshead RM, et al. A randomized clinical trial comparing open and arthroscopic stabilization for recurrent traumatic anterior shoulder instability: two-year follow-up with disease-specific quality-of-life outcomes. J Bone Joint Surg Am 2014;96:353-60. [Crossref] [PubMed]
- Zhang M, Liu J, Jia Y, et al. Risk factors for recurrence after Bankart repair: a systematic review and meta-analysis. J Orthop Surg Res 2022;17:113. [Crossref] [PubMed]
- Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am 2010;92:542-9. [Crossref] [PubMed]
- Waterman B, Owens BD, Tokish JM. Anterior Shoulder Instability in the Military Athlete. Sports Health 2016;8:514-9. [Crossref] [PubMed]
- Calandra JJ, Baker CL, Uribe J. The incidence of Hill-Sachs lesions in initial anterior shoulder dislocations. Arthroscopy 1989;5:254-7. [Crossref] [PubMed]
- Yoshida M, Takenaga T, Chan CK, et al. Altered shoulder kinematics using a new model for multiple dislocations-induced Bankart lesions. Clin Biomech (Bristol, Avon) 2019;70:131-6. [Crossref] [PubMed]
- Hill HA, Sachs MD. The Grooved Defect of the Humeral Head: A frequently unrecognized complication of dislocations of the shoulder joint. Radiology 1940;35:690-700. [Crossref]
- Spatschil A, Landsiedl F, Anderl W, et al. Posttraumatic anterior-inferior instability of the shoulder: arthroscopic findings and clinical correlations. Arch Orthop Trauma Surg 2006;126:217-22. [Crossref] [PubMed]
- Nazzal EM, Herman ZJ, Engler ID, et al. First-time traumatic anterior shoulder dislocation: current concepts. J ISAKOS 2023;8:101-7. [Crossref] [PubMed]
- Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy 2003;19:732-9. [Crossref] [PubMed]
- Nakagawa S, Ozaki R, Take Y, et al. Relationship Between Glenoid Defects and Hill-Sachs Lesions in Shoulders With Traumatic Anterior Instability. Am J Sports Med 2015;43:2763-73. [Crossref] [PubMed]
- Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649-56. [Crossref] [PubMed]
- Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000;16:677-94. [Crossref] [PubMed]
- Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from "engaging/non-engaging" lesion to "on-track/off-track" lesion. Arthroscopy 2014;30:90-8. [Crossref] [PubMed]
- Itoi E. 'On-track' and 'off-track' shoulder lesions. EFORT Open Rev 2017;2:343-51. [Crossref] [PubMed]
- Li RT, Kane G, Drummond M, et al. On-Track Lesions with a Small Distance to Dislocation Are Associated with Failure After Arthroscopic Anterior Shoulder Stabilization. J Bone Joint Surg Am 2021;103:961-7. [Crossref] [PubMed]
- Piasecki DP, Verma NN, Romeo AA, et al. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg 2009;17:482-93. [Crossref] [PubMed]
- Shaha JS, Cook JB, Song DJ, et al. Redefining "Critical" Bone Loss in Shoulder Instability: Functional Outcomes Worsen With "Subcritical" Bone Loss. Am J Sports Med 2015;43:1719-25. [Crossref] [PubMed]
- Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy 2004;20:169-74. [Crossref] [PubMed]
- Hughes JD, Vaswani R, Paras TM, et al. Treatment Algorithm for Recurrent Anterior Shoulder Instability: Putting It All Together. Operative Techniques in Orthopaedics 2021;31:100862. [Crossref]
- Rabinowitz J, Friedman R, Eichinger JK. Management of Glenoid Bone Loss with Anterior Shoulder Instability: Indications and Outcomes. Curr Rev Musculoskelet Med 2017;10:452-62. [Crossref] [PubMed]
- Saliken DJ, Bornes TD, Bouliane MJ, et al. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord 2015;16:164. [Crossref] [PubMed]
- Nakagawa S, Iuchi R, Hanai H, et al. The Development Process of Bipolar Bone Defects From Primary to Recurrent Instability in Shoulders With Traumatic Anterior Instability. Am J Sports Med 2019;47:695-703. [Crossref] [PubMed]
- Sugaya H, Moriishi J, Dohi M, et al. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am 2003;85:878-84. [Crossref] [PubMed]
- Itoi E, Lee SB, Berglund LJ, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am 2000;82:35-46. [Crossref] [PubMed]
- Arenas-Miquelez A, Karargyris O, Graham PL, et al. High correlation between inner and outer glenoid circle diameters and its clinical relevance. Knee Surg Sports Traumatol Arthrosc 2023;31:199-205. [Crossref] [PubMed]
- Baudi P, Righi P, Bolognesi D, et al. How to identify and calculate glenoid bone deficit. Chir Organi Mov 2005;90:145-52. [PubMed]
- Griffith JF, Antonio GE, Tong CW, et al. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol 2003;180:1423-30. [Crossref] [PubMed]
- Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res 2002;65-76. [Crossref] [PubMed]
- Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am 2010;92:133-51. [Crossref] [PubMed]
- Sugaya H. Chapter 14. Instability with Bone Loss. In: Angelo RL, Esch JC, Ryu RK, editors. AANA Advanced Arthroscopy: The Shoulder. Philadelphia: Elsevier; 2010:136-46.
- Green GL, Arnander M, Pearse E, et al. CT estimation of glenoid bone loss in anterior glenohumeral instability: a systematic review of existing techniques. Bone Jt Open 2022;3:114-22. [Crossref] [PubMed]
- Bhatia S, Saigal A, Frank RM, et al. Glenoid diameter is an inaccurate method for percent glenoid bone loss quantification: analysis and techniques for improved accuracy. Arthroscopy 2015;31:608-614.e1. [Crossref] [PubMed]
- Bakshi NK, Cibulas GA, Sekiya JK, et al. A Clinical Comparison of Linear- and Surface Area-Based Methods of Measuring Glenoid Bone Loss. Am J Sports Med 2018;46:2472-7. [Crossref] [PubMed]
- Bois AJ, Fening SD, Polster J, et al. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med 2012;40:2569-77. [Crossref] [PubMed]
- Frank RM, Golijanin P, Vopat BG, et al. Impact of Sagittal Rotation on Axial Glenoid Width Measurement in the Setting of Glenoid Bone Loss. Am J Orthop (Belle Mead NJ) 2018. doi:
10.12788/ajo.2018.0041 .10.12788/ajo.2018.0041 - Lander ST, Liles JL, Kim BI, et al. Comparison of computed tomography and 3D magnetic resonance imaging in evaluating glenohumeral instability bone loss. J Shoulder Elbow Surg 2022;31:2217-24. [Crossref] [PubMed]
- Lansdown DA, Cvetanovich GL, Verma NN, et al. Automated 3-Dimensional Magnetic Resonance Imaging Allows for Accurate Evaluation of Glenoid Bone Loss Compared With 3-Dimensional Computed Tomography. Arthroscopy 2019;35:734-40. [Crossref] [PubMed]
- Sgroi M, Huzurudin H, Ludwig M, et al. MRI Allows Accurate Measurement of Glenoid Bone Loss. Clin Orthop Relat Res 2022;480:1731-42. [Crossref] [PubMed]
- Walter WR, Samim M, LaPolla FWZ, et al. Imaging Quantification of Glenoid Bone Loss in Patients With Glenohumeral Instability: A Systematic Review. AJR Am J Roentgenol 2019;212:1096-105. [Crossref] [PubMed]
- Boileau P, Villalba M, Héry JY, et al. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am 2006;88:1755-63. [Crossref] [PubMed]
- Ahmed I, Ashton F, Robinson CM. Arthroscopic Bankart repair and capsular shift for recurrent anterior shoulder instability: functional outcomes and identification of risk factors for recurrence. J Bone Joint Surg Am 2012;94:1308-15. [Crossref] [PubMed]
- Gouveia K, Rizvi SFH, Dagher D, et al. Assessing Bone Loss in the Unstable Shoulder: a Scoping Review. Curr Rev Musculoskelet Med 2022;15:369-76. [Crossref] [PubMed]
- Shaha JS, Cook JB, Song DJ, et al. “Subcritical” glenoid bone loss increases redislocation rates in primary arthroscopic bankart repair. Orthop J Sport Med 2014. doi:
10.1177/2325967114S00025 .10.1177/2325967114S00025 - Shin SJ, Koh YW, Bui C, et al. What Is the Critical Value of Glenoid Bone Loss at Which Soft Tissue Bankart Repair Does Not Restore Glenohumeral Translation, Restricts Range of Motion, and Leads to Abnormal Humeral Head Position? Am J Sports Med 2016;44:2784-91. [Crossref] [PubMed]
- Shin SJ, Kim RG, Jeon YS, et al. Critical Value of Anterior Glenoid Bone Loss That Leads to Recurrent Glenohumeral Instability After Arthroscopic Bankart Repair. Am J Sports Med 2017;45:1975-81. [Crossref] [PubMed]
- Yang JS, Mehran N, Mazzocca AD, et al. Remplissage Versus Modified Latarjet for Off-Track Hill-Sachs Lesions With Subcritical Glenoid Bone Loss. Am J Sports Med 2018;46:1885-91. [Crossref] [PubMed]
- Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br 2007;89:1470-7. [Crossref] [PubMed]
- Barrow AE, Charles SJ, Issa M, et al. Distance to Dislocation and Recurrent Shoulder Dislocation After Arthroscopic Bankart Repair: Rethinking the Glenoid Track Concept. Am J Sports Med 2022;50:3875-80. [Crossref] [PubMed]
- Pasqualini I, De Cicco FL, Tanoira I, et al. Classic Versus Congruent-Arc Latarjet Procedures. Arthroscopy 2023;39:8-10. [Crossref] [PubMed]
- Itha R, Vaish A, Vaishya R. Hill–Sachs Lesions Revisited. Journal of Arthroscopy and Joint Surgery 2022;9:95-101. [Crossref]
- Gowd AK, Liu JN, Cabarcas BC, et al. Management of Recurrent Anterior Shoulder Instability With Bipolar Bone Loss: A Systematic Review to Assess Critical Bone Loss Amounts. Am J Sports Med 2019;47:2484-93. [Crossref] [PubMed]
- Makhni EC, Tramer JS, Anderson MJJ, et al. Evaluating Bone Loss in Anterior Shoulder Instability. J Am Acad Orthop Surg 2022;30:563-72. [Crossref] [PubMed]
- Pavlov H, Warren RF, Weiss CB Jr, et al. The roentgenographic evaluation of anterior shoulder instability. Clin Orthop Relat Res 1985;153-8. [Crossref] [PubMed]
- Funakoshi T, Hartzler RU, Stewien E, et al. Hill-Sachs Lesion Classification by the Glenoid Track Paradigm in Shoulder Instability: Poor Agreement Between 3-Dimensional Computed Tomographic and Arthroscopic Methods. Arthroscopy 2019;35:1743-9. [Crossref] [PubMed]
- Cho SH, Cho NS, Rhee YG. Preoperative analysis of the Hill-Sachs lesion in anterior shoulder instability: how to predict engagement of the lesion. Am J Sports Med 2011;39:2389-95. [Crossref] [PubMed]
- Ozaki R, Nakagawa S, Mizuno N, et al. Hill-sachs lesions in shoulders with traumatic anterior instability: evaluation using computed tomography with 3-dimensional reconstruction. Am J Sports Med 2014;42:2597-605. [Crossref] [PubMed]
- Gyftopoulos S, Beltran LS, Bookman J, et al. MRI Evaluation of Bipolar Bone Loss Using the On-Track Off-Track Method: A Feasibility Study. AJR Am J Roentgenol 2015;205:848-52. [Crossref] [PubMed]
- Khan S, Shanmugaraj A, Faisal H, et al. Variability in quantifying the Hill-Sachs lesion: A scoping review. Shoulder Elbow 2023;15:465-83. [PubMed]
- Assunção JH, Gracitelli ME, Borgo GD, et al. Tomographic evaluation of Hill-Sachs lesions: is there a correlation between different methods of measurement? Acta Radiol 2017;58:77-83. [Crossref] [PubMed]
- Schneider AK, Hoy GA, Ek ET, et al. Interobserver and intraobserver variability of glenoid track measurements. J Shoulder Elbow Surg 2017;26:573-9. [Crossref] [PubMed]
- Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am 1984;66:159-68. [Crossref] [PubMed]
- Di Giacomo G, de Gasperis N. Measuring Bone Loss in the Unstable Shoulder: Understanding and Applying the Track Concept. Sports Med Arthrosc Rev 2020;28:153-8. [Crossref] [PubMed]
- Yamamoto N, Shinagawa K, Hatta T, et al. Peripheral-Track and Central-Track Hill-Sachs Lesions: A New Concept of Assessing an On-Track Lesion. Am J Sports Med 2020;48:33-8. [Crossref] [PubMed]
- Locher J, Wilken F, Beitzel K, et al. Hill-Sachs Off-track Lesions as Risk Factor for Recurrence of Instability After Arthroscopic Bankart Repair. Arthroscopy 2016;32:1993-9. [Crossref] [PubMed]
- Dyrna FGE, Ludwig M, Imhoff AB, et al. Off-track Hill-Sachs lesions predispose to recurrence after nonoperative management of first-time anterior shoulder dislocations. Knee Surg Sports Traumatol Arthrosc 2021;29:2289-96. [Crossref] [PubMed]
- Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med 2006;34:979-85. [Crossref] [PubMed]
- Zimmermann SM, Scheyerer MJ, Farshad M, et al. Long-Term Restoration of Anterior Shoulder Stability: A Retrospective Analysis of Arthroscopic Bankart Repair Versus Open Latarjet Procedure. J Bone Joint Surg Am 2016;98:1954-61. [Crossref] [PubMed]
Cite this article as: Herman ZJ, Nazzal EM, Keeling L, Reddy RP, Como M, Hughes JD, Lin A. Bipolar bone loss and distance to dislocation. Ann Joint 2024;9:7.


