
Inflammatory synovial biomarkers and state of the tibiofemoral joint in the post-surgical settings: a narrative review
Introduction
Background
After acute injury to the structures of the knee, including ligamentous (1), meniscal (2), and cartilaginous (3) injury, surgical intervention is a common method to repair or reconstruct the damaged tissues to prevent knee tissue degradation and osteoarthritis (OA). The initial injury and subsequent surgery can cause the joint to enter a proinflammatory state (1). The concentration of specific inflammatory and cartilage breakdown related biomarkers can be measured in the synovial fluid of the joint (4-6). These proinflammatory biomarkers may contribute to early progression of knee OA and increased knee inflammation which can cause decreased long-term patient outcomes (4,6). Some common biomarkers include interleukin, matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), and monocyte chemoattractant proteins (MCPs) (7). Interleukin is known to induce several MMPs and aggrecanases and suppress proteoglycan synthesis leading to cartilage degradation (8). MMPs are responsible for extracellular matrix degradation of cartilage (7). VEGF is an angiogenesis signal factor responsible for blood vessel recruitment and can increase inflammation and macrophage recruitment (9). MCPs are secreted in response to stimulation by inflammatory biomarkers and recruit monocytes which facilitates the inflammatory response (10). These main inflammatory synovial fluid biomarkers and associated molecules along with others are correlated to OA and inflammation and can be used to track the state of the knee after injury and surgery (Table 1).
Table 1
| Number | Biomarker name | Abbreviation | Function | Reference |
|---|---|---|---|---|
| 1 | Aggrecanases-1 | ADAMTS-4 | Participate in the cleavage of aggrecans in the extracellular matrix of cartilage, elevated in patients with osteoarthritis | (11,12) |
| 2 | Sulfated glycosaminoglycan | sGAG | Function in cartilage homeostasis, released during the degradation of cartilage | (13-15) |
| 3 | Monocyte chemotactic protein-1 | MCP-1 | Stimulated in response to inflammatory biomarkers, recruit monocytes, memory T-cells, and dendritic cells | (10,16,17) |
| 4 | Interleukin-6 | IL-6 | Cytokine secreted by macrophages with proinflammatory effects, contributes to cartilage matrix breakdown | (13,16,18-20) |
| 5 | Interleukin-1 beta | IL-1β | Mediator of inflammatory response, released by macrophages | (16,19) |
| 6 | Carboxy-terminal telopeptides of type II collagen | CTX-II | Marker of cartilage breakdown | (19,21) |
| 7 | Myostatin | Induces muscle atrophy via the ubiquitin-proteasome pathway | (22,23) | |
| 8 | Transforming growth factor beta | TGF-β | Potentially induces muscle atrophy and weakness via the ubiquitin-proteasome pathway | (22,23) |
| 9 | Aggrecan neoepitope fragment | ARGS | Aggrecan measured in the synovial fluid signaling cleavage of aggrecan in the cartilage | (13,21) |
| 10 | Interleukin-8 | IL-8 | Promotes release of metalloproteinase and aggregation of neutrophil | (20) |
| 11 | Interleukin-10 | IL-10 | Anti-inflammatory cytokine, used for downregulation of other inflammatory cytokines | (16) |
| 12 | Tumor necrosis factor alpha | TNF-α | Cytokine produced by immune response, usually present in the acute phase, and can induce apoptosis | (13,16,20,24) |
| 13 | Cartilage oligomeric matrix protein | COMP | Biomarker suggestive of cartilage breakdown | (13) |
| 14 | Vascular endothelial growth factor | VEGF | Angiogenesis growth factor | (9,16,18,24) |
After the initial traumatic response to an injury, the secondary trauma caused by surgery can lead to persistent and long-term inflammatory biomarkers that may be different from the initial biomarker composition that occurs initially after injury (19,25). It is believed that these inflammatory biomarkers lead to decrease patient outcomes and OA progression by stimulating cartilage degradation (3,26). Synovial biomarkers typically appear earlier than OA symptoms, so determining the most important and relevant inflammatory biomarkers could be critical for developing improved post-surgical knee treatment options. To our knowledge, no other studies report on the various synovial fluid biomarkers of the knee in the post-surgical state of the knee.
Rational and knowledge gap
Although surgical techniques have improved for various knee injuries, the prevalence of the postoperative pain and OA is still high (27). Surgeries like anterior cruciate ligament (ACL) reconstruction (28) and meniscal repair (29) have been reported to improve knee biomechanics and stability and improve outcomes for patients. However, OA and other complications still occur, as evidenced by long-term outcomes and biomarkers in the synovial fluid of the joint. It is unknown whether the degradation of the knee joint after surgery is due to the inflammatory biomarkers associated with injury and trauma, non-biomechanical repairs and reconstruction of knee structures, or other factors including patient age and time between injury and surgery (30).
Objective
The objective of this narrative review is to analyze the current literature relating to synovial fluid biomarkers of the knee relating to the post-surgical setting. By obtaining an improved understanding of the synovial fluid state of the knee after surgery, physicians and care providers can develop patient specific treatment options to improve short- and long-term patient outcomes and decrease knee tissue degradation after knee surgery. Management strategies to decrease negative synovial biomarkers may help slow the progression of OA and inflammation of the knee joint, while preserving the beneficial biomarkers (31). Our hypothesis is that the post-surgical knee setting will have different concentrations of synovial fluid biomarkers than the pre-surgical setting. We present this article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-56/rc).
Methods
A narrative review was conducted of the literature reporting on synovial fluid biomolecular markers associated with the post-surgical knee. The literature review was performed using sources from PubMed and MEDLINE. All studies were cross referenced to find additional sources. Studies were initially screened by analysis of the abstract and then further analyzed if the abstract was relevant to the topic of choice. Studies were included that contained information for synovial fluid biomarkers in the post-surgical knee including studies discussing ways to manage these biomarkers. There was no exclusion based on the date of publication. The summary of the search methods can be found in Table 2.
Table 2
| Items | Specification |
|---|---|
| Date of search | 7/10/23 |
| Databases and other sources searched | PubMed and MEDLINE |
| Search terms used | (inflammatory biomarkers OR knee biomarkers OR inflammatory markers OR inflammatory biomarker inhibition) AND (knee surgery OR knee osteoarthritis OR ACL reconstruction OR anterior cruciate ligament reconstruction) |
| Timeframe | 2006–2023 |
| Inclusion and exclusion criteria | Inclusion: full text peer reviewed articles, English |
| Exclusion: single case reports | |
| Selection process | Article selection was performed by determining relevance from title and abstract and was performed independently by two co-authors (L.V.T. and M.I.K.). Consensus was obtained by analyzing the full text |
Post-surgical inflammatory state
Complications, including OA, are common after knee injury and surgery. A study by Barenius et al. (32) reported that at 14 years follow-up, 57% of patients had OA after having an ACL reconstruction compared to 18% on the contralateral side. It has been shown that acute knee injuries lead to an increase in inflammatory biomarkers (15,33), but that surgery may induce a secondary hit that further increases the prevalence of inflammatory synovial biomarkers. Knee surgery aims to repair or reconstruct deficient knee structures to improve stability and kinematics to delay OA compared to leaving injuries untreated (34). The cause of OA after surgery is still unknown, but it could be due to any number of factors including improper restoration of knee biomechanics, reduced knee strength postoperatively, or inflammatory biochemical markers. This paper will look specifically at the inflammatory biomarkers present before and after knee surgery to attempt to gain a better understanding of synovial fluid biomarker progression from initial injury to years after surgery.
Preoperative synovial biochemical markers
Preoperative synovial biomarkers can help determine the state of the knee prior to surgery and determine if any predictive inflammatory biomarkers are already present from the initial injury. Certain synovial biomarkers have already been reported to be associated with cartilage breakdown and OA progression, like macrophages and cytokines. The presence, or absence, of certain synovial biomarkers related to the breakdown of cartilage and inflammatory signals could offer insights into determination of surgery, preoperative planning, and postoperative rehabilitation and guidelines.
Some knee cartilage treatments, like autologous chondrocyte implantation (ACI), attempt to treat full thickness articular cartilage defects by using patient specific lab grown chondrocytes. A study by Wright et al. (12) measured preoperative synovial biomarkers before ACI surgery and reported that a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4), which is an aggrecanase that plays a key role in early cartilage destruction, had elevated activity in the synovial fluid of patients who had decreased clinical outcomes (11). This suggests that ADAMTS-4 might be a potential biomarker showing that OA has already progressed too far and surgical treatment may not be beneficial. Measuring levels of ADAMTS-4 may be a beneficial diagnostic tool for surgical indications and a predictor of positive patient outcomes. Additionally, it is possible that ADAMTS-4 could be a future therapeutic target to delay the onset of OA.
Another preoperative synovial fluid biomarker found to be related to poor patient outcomes after surgery is sulfated glycosaminoglycan (sGAG), a molecule released during cartilage breakdown. A study by Amano et al. (14) reported that elevated level of sGAG at the time of surgery resulted in decreased patient outcomes at a 3-year follow-up. This is suggestive of preoperative screening to potentially be used as a predictor of patient outcomes. This study did mention the limitation of not having an improved understanding of the timeline progression of sGAG due to only testing patient synovial fluid samples prior to surgery and at 3 years postoperatively. Future studies should look at biomarker levels immediately after injury, before surgery, after surgery and at more time points along the rehabilitation process. They also suggested analyzing other biomarkers, like hyaluronic acid and lubricin.
Another potential application of analyzing preoperative synovial fluid biomarkers could be to determine how severe a cartilage injury may be. Although this would not replace true diagnostic determination, it could be a useful tool to augment a physician’s diagnostic capabilities. A study by Cuéllar et al. (16) reported on preoperative synovial biomarkers before knee arthroscopic surgery and determined that higher levels of monocyte chemotactic protein 1 (MCP-1) and interleukin 6 (IL-6) were the strongest predictors of more severe cartilage damage during intraoperative inspection. Both MCP-1 and IL-6 have been characterized as inflammatory biomarkers related to cartilage breakdown and involved in injury response.
Preoperative synovial fluid biomarkers have the potential to help physicians weigh the risks and benefits of surgical intervention and may assist in defining specific pro-inflammatory modulators to target in the pre- and post-operative setting to improve outcomes. Certain biomarkers have been reported to help predict whether surgery will be beneficial for patient outcomes. Low levels or absence of ADAMTS-4 and sGAG can be useful in predicting whether ACI or other knee surgeries will be a beneficial surgery to help prevent future cartilage degradation. High levels of these biomarkers preoperatively have been shown to correlate with decreased patient outcomes regarding pain, function, and progression of OA (12,14). Other biomarkers, like MCP-1 and IL-6, can help physicians determine the extent of cartilage damage prior to surgery. Having a better understanding of the status of the joint, like if severe cartilage damage is present, prior to surgery may be beneficial for surgical planning. These markers can be used in conjunction with other preoperative risk factors to provide a more in-depth patient analysis prior to surgical intervention. More research needs to be performed to incorporate the testing of more biomarkers and at more timepoints to gain a deeper understanding of potential preoperative indicators for patient outcomes.
Early postoperative biomarkers
Many synovial fluid biomarkers are expressed after an acute injury and continue up until and after surgery occurs. Determining the effect of early postoperative biomarkers requires comparing preoperative biomarker levels to postoperative biomarker levels. Surgery often creates a secondary hit of inflammatory biomarkers, possibly leading to the presence of new biomarkers and further expounding the effects of the inflammatory biomarkers from the initial injury (17). Understanding the impact and implication of this secondary hit of inflammation to the knee joint could be beneficial for understanding how surgery might contribute to patient outcomes and the progression of OA.
Understanding how specific inflammatory and cartilage breakdown biomarkers, like IL-1β, IL-6, and C-terminal cross-linked telopeptides of type II collagen (CTX-II), compare between preoperative levels and early postoperative levels can help determine which biomarkers are related to the secondary hit of surgery. One study by Hunt et al. (19) reported that inflammatory cytokine IL-1β and IL-6 levels were higher 1-week and 1-month postoperative timeframe than they were 1 week post injury and immediately prior to surgery (mean =23 days post injury). They also reported that CTX-II, a marker of cartilage breakdown, was significantly increased at 4 weeks postoperative suggesting the second hit of an inflammatory response after ACL reconstruction leading to cartilage breakdown. These observations could indicate that the increased inflammatory response caused by surgery could be contributing to increased cartilage breakdown. Although the patients had different timelines between their initial injuries and their date of surgery, having reported significantly increased cartilage breakdown biomarkers at 4 weeks after surgery could indicate that this increase in breakdown is caused by the surgery rather than by the initial injury.
Another aspect of the inflammatory and biomarker response to surgery involves muscle atrophy and weakness following surgery. A study by Mendias et al. (23) reported that myostatin and transforming growth factor-β (TGF-β) levels were significantly increased in the early postoperative period compared to preoperative levels. Elevated myostatin and TGF-β levels are indicated for increased muscle atrophy and weakness (22). In the early postoperative setting, elevated levels of these biomarkers potentially lead to increased quad atrophy and decreased strength which limits recovery, decreases knee stability, and could potentially delay return to sport or activity. Since strength is important for knee stability, a chronic decrease in strength after surgery compared to before surgery could potentially contribute to the increase in inflammatory biomarkers and cartilage breakdown. Patient specific rehabilitation strength protocol could be a way to decrease proinflammatory biomarkers after surgery (35). Finding a way to decrease the muscle atrophy and weakness after surgery could help patients regain strength back to preoperative levels helping to prevent losses in knee stability.
To determine which biomarkers are specifically part of the early postoperative phase, a longitudinal study must be performed evaluating samples immediately before and after surgery and samples taken intermittently for the months and years following surgery. A study by Struglics et al. (21) reported that inflammatory cytokine biomarkers and aggrecan ARGS (amino acids alanine, arginine, glycine, serine) neoepitope peak immediately after surgery but then typically decrease significantly by 30 weeks and show no difference to reference samples. Knowing which biomarkers are present immediately after surgery but decrease as time progresses is important to analyze to determine if any short-term biomarkers correlate with decreased patient outcomes or an increased prevalence of OA.
Acute knee injuries increase pro-inflammatory biomarkers and surgical treatment further increases these same biomarkers, like IL-1β, IL-6, CTX-II (19,21). These synovial biomarkers have been reported to be significantly increased at 1 month postoperatively compared to preoperative levels suggesting a second hit to the knee resulting in a prolonged and increased inflammatory response and increased cartilage breakdown. Other biomarkers, like myostatin and TGF-β increase muscle atrophy and contribute to prolonged muscle weakness and knee instability, which can lead to further knee inflammation (23). Many other biomarkers are increased in the first week postoperatively but decrease by the end of the first month. Currently, no studies have reported if the increase of these specific early postoperative biomarkers has any significant correlation with decreased patient outcomes or the progression and development of postoperative OA.
Extended postoperative biomarkers
Extended postoperative synovial biomarkers remain active for months to years postoperatively. When determining inflammatory biomarkers associated with patient outcomes and OA, analyzing the long-term biomarkers offers the most robust form of measurement (20,36). To best understand the extended postoperative biomarkers associated with knee surgery, biomarker must be tracked over time starting immediately after the injury occurs (13). Understanding how these biomarkers relate to the preoperative and early postoperative biomarkers is also important to determine the correlation between the progression of the biomarkers in relation to the injury and progression of OA (30).
Interleukin is a common inflammatory biomarker that is typically present during an inflammatory response. A study by Larsson et al. (20) looked at four patient groups with ACL injuries, one group underwent early ACL reconstruction (10 weeks post injury), one group underwent delayed ACL reconstruction (after 10 weeks post injury), one group underwent non-ACL arthroscopic surgery, and one group underwent rehabilitation only with no surgery. The synovial fluid biomarkers of these patients were measured at various intervals from baseline to 5 years post injury. Synovial fluid inflammatory biomarkers IL-6, IL-8, IL-10, and tumor necrosis factor (TNF) were increased at various point postoperatively in the patients who underwent ACL reconstruction (early and delayed) compared to those in rehabilitation alone or with non-ACL arthroscopic surgery. Although both TNF and IL-6 were elevated at 8 months post operatively, IL-6 was the only biomarker still significantly increased in the ACL reconstruction patients compared to the rehabilitation only patients at the 5-year mark. This is comparable to an increased IL-6 biomarker in patients in the early postoperative timeframe.
A comparison between ACL deficient (ACL-D) and ACL reconstruction (ACL-R) knees offers an opportunity to determine differences between knee synovial biomarker levels for surgical and non-surgical approaches. A systematic review by Harkey et al. (36) compared ACL-deficient knees to ACL reconstruction knees and their associated biomarkers. They reported that ACL-D knees have more collagen degradation and less inflammatory biomarkers compared to ACL-R knees. ACL-D knees continue to have deficiencies in stability and proper knee kinematics which could contribute to the continued cartilage damage and degeneration. ACL-R knees have improved stability and kinematics; however, the inflammatory response associated with surgery could lead to OA. This is consistent with the findings that knee surgery causes a secondary hit to the inflammatory biomarkers of the knee.
Although some inflammatory biomarkers may not show significant differences long term, signs of degradation and OA may still be present. A study by Åhlén et al. (13) reported that at 8 years postoperatively, there was no significant difference in inflammatory biomarkers IL-1β, IL-6, TNF-α, sGAG, ARGS-aggrecan, or cartilage oligomeric matrix protein (COMP) between the surgical knee and contralateral knee. However, they did report that the surgical knees had more osteoarticular changes and meniscal and cartilage damage compared to the nonsurgical knee. This could indicate that at the 8-year mark, some of the biomarkers are no longer elevated due to surgery or there could be other biomarkers that were not analyzed in this study that are potentially critical to the progression of OA (4). This study validates the need for more robust testing for biomarkers in the progression of knee OA after surgery. All the patients from this study were confirmed to have intact ACL grafts suggesting that ACL reconstruction might not restore normal biomechanics or that the surgical procedure itself contributes to the degradation of the joint tissues.
The results from long-term studies on knee inflammatory biomarkers suggest that the prevalence of knee OA is greater in patients who have undergone previous knee surgery (30,37). Typically, surgery attempts to restore native knee biomechanics and improve stability of the joint to prevent damages caused by instability due to injury. Although very few inflammatory biomarkers like IL-6 and TNF are present years after surgery, there is clearly extra degradation of the knee cartilage compared to uninjured knees. For future studies, testing more synovial biomarkers and a better testing schedule timeline (Figure 1) in relation to knee injury and surgery could help develop an improved understanding of these biomarkers. Having an improved understanding of the biomarkers could help physicians determine the cause of postoperative OA and explore treatment options for these patients.
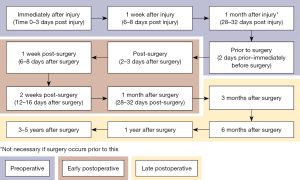
Inflammatory biomarker control
Controlling inflammatory biomarkers may be a potential therapeutic intervention for patients undergoing knee surgery. First, proper identification of the biomarkers in the preoperative, early postoperative, and late postoperative timelines that correlate with decreased patient outcomes or the progression of OA is critical. Next, methods to decrease or inhibit those inflammatory biomarkers need to be developed to slow their detrimental effects. Some investigation has been done into interleukin inhibitors (38) and non-steroidal anti-inflammatory drugs (NSAIDs) with limited success. Investigation into possible inhibitors cannot be done until the specific negative synovial biomarkers are identified.
Currently, NSAID use is recommended for OA patients to decrease pain and improve function (39). Many inflammatory biomarkers are associated with pain, a decrease in function, and OA. A study by Gallelli et al. (24) reported a decrease in inflammatory biomarkers IF-6, TNF-α, and VEGF for patients indicated for knee arthroplasty when using different kinds of NSAIDs for a 2-week period. Notably, the patients also reported an improvement in The Western Ontario and McMaster University (WOMAC) score following the treatment with NSAIDs. The limitation of this study was that the progression of OA is a long-term process and this study was only performed over a 2-week period before surgery and could not determine other possible risk factors of taking NSAIDs for a longer period. A study by Osani et al. (40) reported that patient pain and functional improvements peaked at around 2 weeks after starting NSAID use but steadily decreased after the 4-week mark. Additionally, after 4 weeks, cardiovascular and gastrointestinal adverse events continually increased. NSAIDs may be beneficial in short-term relief but may not be a viable solution for long-term care.
The future of OA therapeutic treatment could involve anti-inflammatory molecules, like selective IL-1 inhibitors, that may help decrease the concentration of inflammatory biomarkers. A clinical study by Kraus et al. (41) reported that the use of IL-1 receptor antagonist two weeks after a severe knee injury resulted in improved patient outcomes for pain and function over a 2-week interval after treatment. This study suggests that the use of receptor agonists or inhibitors of knee inflammatory biomarkers may be a viable solution to improve patient outcomes. A systematic review by Aman et al. (42) reported that a selective IL-1 inhibitor was effective for reducing levels of the proinflammatory biomarker IL-1β in the acute phase after knee injury. This reduction in IL-1β helped to slow OA progression and cartilage damage in animal models. However, these therapies had a diminished effect long-term, emphasizing the need for a more effective method for drug delivery and long-term studies. This review does mention the lack of literature surrounding IL-1 inhibitors to treat OA, citing the clinical study as the only clinical study for IL-1 inhibition. This emphasizes a need for more research into this subject to explore long-term outcomes and the inhibition of more inflammatory biomarkers.
In the future, postoperative therapeutic treatment for inflammatory synovial fluid biomarkers could become a standard of care to improve patient outcomes. Research is in progress of evaluating interleukin inhibitors to treat pain and improve function; however, more needs to be done to determine the long-term effects (24,38,42). NSAIDs may also help reduce inflammatory biomarkers and there is potential that NSAIDs can be used with other inhibitors to decrease the inflammatory state of the knee. In the future, this could mean that a combination of NSAIDs and other treatments might be required to decrease the pro-inflammatory biomarkers and cartilage breakdown biomarkers. Inflammatory biomarkers may differ slightly between patients and each patient’s specific synovial fluid analysis would be beneficial to offer a specific treatment to counteract or limit the biomarkers that are present in their synovial fluid.
Discussion
The main findings of this study suggest that there are specific inflammatory synovial biomarkers associated with the post-surgical setting of the knee; however, there is no consensus on which biomarkers are not beneficial and how to counteract their effects. Synovial biomarkers like IL-1β, IL-6, IL-8, IL-10, TGF-β, TNF, and VEGF have all been indicated to be present after injury and knee surgery (Figure 2). These biomarkers are associated with increased knee inflammation and cartilage matrix degradation. Although there is no consensus on the direct association of these biomarkers with specific time periods or patient outcomes, there is a high probability that these biomarkers and others are associated with a post-surgical response. Continued research into testing and analyzing more biomarkers should be performed to gain a deeper understanding of the processes that are going on during knee injury, surgery, and rehabilitation.
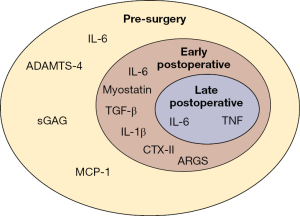
From the current research and reported literature, knee surgery to repair or reconstruct structures to increase stability and correct kinematics can improve patient outcomes. However, surgery can induce a secondary inflammatory response that can contribute to decreased patient outcomes. With a better understanding of the associated biomarkers, treatment and rehabilitation, knee surgery treatment could expand into treating the bodily response, specifically released synovial biomarkers, after injury and surgery. In the future, this could be used to help prevent knee degeneration and arthritis by treating the biomarkers which appear before the main physical symptoms appear.
Current limitations to understanding the effects of postoperative synovial fluid inflammatory biomarkers include the inability to specifically differentiate post-injury and post-surgery biomarkers. Many of the inflammatory and cartilage degeneration biomarkers are similar between post-injury and post-surgery, more should be done to determine differences in concentration and if there are biomarkers present after surgery that are not present at any other points. Additionally, altered knee biomechanics, even after reconstruction and patient rehabilitation, need to be studied in more depth to determine the true cause of decreased patient outcomes.
The future research should explore more biomarkers that could be associated with the overall inflammatory state of the knee caused by injury and surgery. It should also explore in more depth the timing and the progression of different biomarkers as they relate to the injury, surgery, and the post operative rehabilitation. Finally, more review into the possible treatment options to decrease or counteract the indicated biomarkers is recommended. Further research into this topic will hopefully allow physicians to improve their treatment options for patients and help slow down the progression of the long-term negative outcome of OA after knee injury and surgery.
Conclusions
Synovial fluid biomarkers in the post-surgical knee setting may contribute to decreased patient outcomes and the progression of knee tissue degradation. There is no current consensus on which of these biomarkers are the most detrimental or associated with decreased patient outcomes. With an improved understanding of the individual biomarkers, potential personalized therapeutic treatment could be used by physicians in the future to improve patient outcomes after surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Inflammation of the Tibiofemoral Joint: Inflammatory Mediators, Treatment, and Long-Term Effects”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-56/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-56/coif). The series “Inflammation of the Tibiofemoral Joint: Inflammatory Mediators, Treatment, and Long-Term Effects” was commissioned by the editorial office without any funding or sponsorship. N.N.D. served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from August 2022 to July 2024. J.C. is a consultant for Smith & Nephew; has received educational support from Medwest Associated; and has received research support from RTI. G.M. is a consultant for Smith & Nephew and IBSA; is on the editorial board for JBJS and Arthroscopy; and is a committee member for ISAKOS. R.F.L. is a consultant for Ossur, Smith & Nephew, and Responsive Arthroscopy; collects royalties from Ossur, Smith & Nephew, Elsevier, and Arthrex; has research grants from Ossur, Smith & Nephew, AANA, AOSSM; is on the committees for ISAKOS, AOSSM, AANA; and is on the editorial boards of AJSM, JEO, KSSTA, JKS, JISPT and OTSM. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Evers BJ, Van Den Bosch MHJ, Blom AB, et al. Post-traumatic knee osteoarthritis; the role of inflammation and hemarthrosis on disease progression. Front Med (Lausanne) 2022;9:973870. [Crossref] [PubMed]
- Bradley PX, Thomas KN, Kratzer AL, et al. The Interplay of Biomechanical and Biological Changes Following Meniscus Injury. Curr Rheumatol Rep 2023;25:35-46. [Crossref] [PubMed]
- Kumavat R, Kumar V, Malhotra R, et al. Biomarkers of Joint Damage in Osteoarthritis: Current Status and Future Directions. Mediators Inflamm 2021;2021:5574582. [Crossref] [PubMed]
- Boffa A, Merli G, Andriolo L, et al. Synovial Fluid Biomarkers in Knee Osteoarthritis: A Systematic Review and Quantitative Evaluation Using BIPEDs Criteria. Cartilage 2021;13:82S-103S. [Crossref] [PubMed]
- Bondeson J, Wainwright SD, Lauder S, et al. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006;8:R187. [Crossref] [PubMed]
- Braaten JA, Banovetz MT, DePhillipo NN, et al. Biomarkers for Osteoarthritis Diseases. Life (Basel) 2022;12:1799. [Crossref] [PubMed]
- Clair AJ, Kingery MT, Anil U, et al. Alterations in Synovial Fluid Biomarker Levels in Knees With Meniscal Injury as Compared With Asymptomatic Contralateral Knees. Am J Sports Med 2019;47:847-56. [Crossref] [PubMed]
- Vincent TL. IL-1 in osteoarthritis: time for a critical review of the literature. F1000Res 2019;8:F1000 Faculty Rev-934.
- Haraden CA, Huebner JL, Hsueh MF, et al. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther 2019;21:146. [Crossref] [PubMed]
- Ni F, Zhang Y, Peng X, et al. Correlation between osteoarthritis and monocyte chemotactic protein-1 expression: a meta-analysis. J Orthop Surg Res 2020;15:516. [Crossref] [PubMed]
- Li T, Peng J, Li Q, et al. The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis. Biomolecules 2022;12:959. [Crossref] [PubMed]
- Wright KT, Kuiper JH, Richardson JB, et al. The Absence of Detectable ADAMTS-4 (Aggrecanase-1) Activity in Synovial Fluid Is a Predictive Indicator of Autologous Chondrocyte Implantation Success. Am J Sports Med 2017;45:1806-14. [Crossref] [PubMed]
- Åhlén M, Roshani L, Lidén M, et al. Inflammatory cytokines and biomarkers of cartilage metabolism 8 years after anterior cruciate ligament reconstruction: results from operated and contralateral knees. Am J Sports Med 2015;43:1460-6. [Crossref] [PubMed]
- Amano K, Huebner JL, Stabler TV, et al. Synovial Fluid Profile at the Time of Anterior Cruciate Ligament Reconstruction and Its Association With Cartilage Matrix Composition 3 Years After Surgery. Am J Sports Med 2018;46:890-9. [Crossref] [PubMed]
- Catterall JB, Stabler TV, Flannery CR, et al. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther 2010;12:R229. [Crossref] [PubMed]
- Cuéllar VG, Cuéllar JM, Kirsch T, et al. Correlation of Synovial Fluid Biomarkers With Cartilage Pathology and Associated Outcomes in Knee Arthroscopy. Arthroscopy 2016;32:475-85. [Crossref] [PubMed]
- Kingery MT, Anil U, Berlinberg EJ, et al. Changes in the Synovial Fluid Cytokine Profile of the Knee Between an Acute Anterior Cruciate Ligament Injury and Surgical Reconstruction. Am J Sports Med 2022;50:451-60. [Crossref] [PubMed]
- Ding J, Niu X, Su Y, et al. Expression of synovial fluid biomarkers in patients with knee osteoarthritis and meniscus injury. Exp Ther Med 2017;14:1609-13. [Crossref] [PubMed]
- Hunt ER, Jacobs CA, Conley CE, et al. Anterior cruciate ligament reconstruction reinitiates an inflammatory and chondrodegenerative process in the knee joint. J Orthop Res 2021;39:1281-8. [Crossref] [PubMed]
- Larsson S, Struglics A, Lohmander LS, et al. Surgical reconstruction of ruptured anterior cruciate ligament prolongs trauma-induced increase of inflammatory cytokines in synovial fluid: an exploratory analysis in the KANON trial. Osteoarthritis Cartilage 2017;25:1443-51. [Crossref] [PubMed]
- Struglics A, Larsson S, Kumahashi N, et al. Changes in Cytokines and Aggrecan ARGS Neoepitope in Synovial Fluid and Serum and in C-Terminal Crosslinking Telopeptide of Type II Collagen and N-Terminal Crosslinking Telopeptide of Type I Collagen in Urine Over Five Years After Anterior Cruciate Ligament Rupture: An Exploratory Analysis in the Knee Anterior Cruciate Ligament, Nonsurgical Versus Surgical Treatment Trial. Arthritis Rheumatol 2015;67:1816-25. [Crossref] [PubMed]
- Buehring B, Binkley N. Myostatin--the holy grail for muscle, bone, and fat? Curr Osteoporos Rep 2013;11:407-14. [Crossref] [PubMed]
- Mendias CL, Lynch EB, Davis ME, et al. Changes in circulating biomarkers of muscle atrophy, inflammation, and cartilage turnover in patients undergoing anterior cruciate ligament reconstruction and rehabilitation. Am J Sports Med 2013;41:1819-26. [Crossref] [PubMed]
- Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage 2013;21:1400-8. [Crossref] [PubMed]
- Li H, Chen C, Chen S. Posttraumatic knee osteoarthritis following anterior cruciate ligament injury: Potential biochemical mediators of degenerative alteration and specific biochemical markers. Biomed Rep 2015;3:147-51. [Crossref] [PubMed]
- Subburaman M, Edderkaoui B. Evaluation of CCL21 role in post-knee injury inflammation and early cartilage degeneration. PLoS One 2021;16:e0247913. [Crossref] [PubMed]
- Keays SL, Newcombe PA, Bullock-Saxton JE, et al. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med 2010;38:455-63. [Crossref] [PubMed]
- Goldsmith MT, Jansson KS, Smith SD, et al. Biomechanical comparison of anatomic single- and double-bundle anterior cruciate ligament reconstructions: an in vitro study. Am J Sports Med 2013;41:1595-604. [Crossref] [PubMed]
- LaPrade CM, Jansson KS, Dornan G, et al. Altered tibiofemoral contact mechanics due to lateral meniscus posterior horn root avulsions and radial tears can be restored with in situ pull-out suture repairs. J Bone Joint Surg Am 2014;96:471-9. [Crossref] [PubMed]
- Cinque ME, Dornan GJ, Chahla J, et al. High Rates of Osteoarthritis Develop After Anterior Cruciate Ligament Surgery: An Analysis of 4108 Patients. Am J Sports Med 2018;46:2011-9. [Crossref] [PubMed]
- Holm PM, Juhl CB, Culvenor AG, et al. The Effects of Different Management Strategies or Rehabilitation Approaches on Knee Joint Structural and Molecular Biomarkers Following Traumatic Knee Injury: A Systematic Review of Randomized Controlled Trials for the OPTIKNEE Consensus. J Orthop Sports Phys Ther 2023;53:1-22. [Crossref] [PubMed]
- Barenius B, Ponzer S, Shalabi A, et al. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med 2014;42:1049-57. [Crossref] [PubMed]
- Nieboer MF, Reijman M, Wesdorp MA, et al. Improved Understanding of the Inflammatory Response in Synovial Fluid and Serum after Traumatic Knee Injury, Excluding Fractures of the Knee: A Systematic Review. Cartilage 2023;14:198-209. [Crossref] [PubMed]
- Steineman BD, LaPrade RF, Santangelo KS, et al. Early Osteoarthritis After Untreated Anterior Meniscal Root Tears: An In Vivo Animal Study. Orthop J Sports Med 2017;5:2325967117702452. [Crossref] [PubMed]
- Nambi G, Abdelbasset WK, Alrawail SM, et al. Effects of isokinetic knee muscle training on bone morphogenetic proteins and inflammatory biomarkers in post-traumatic osteoarthritis after anterior cruciate ligament injury: A randomized trial. J Rehabil Med 2020;52:jrm00098. [Crossref] [PubMed]
- Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage 2015;23:1-12. [Crossref] [PubMed]
- Smith MV, Nepple JJ, Wright RW, et al. Knee Osteoarthritis Is Associated With Previous Meniscus and Anterior Cruciate Ligament Surgery Among Elite College American Football Athletes. Sports Health 2017;9:247-51. [Crossref] [PubMed]
- Furman BD, Mangiapani DS, Zeitler E, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther 2014;16:R134. [Crossref] [PubMed]
- Adatia A, Rainsford KD, Kean WF. Osteoarthritis of the knee and hip. Part II: therapy with ibuprofen and a review of clinical trials. J Pharm Pharmacol 2012;64:626-36. [Crossref] [PubMed]
- Osani MC, Vaysbrot EE, Zhou M, et al. Duration of Symptom Relief and Early Trajectory of Adverse Events for Oral Nonsteroidal Antiinflammatory Drugs in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 2020;72:641-51. [Crossref] [PubMed]
- Kraus VB, Birmingham J, Stabler TV, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial (NCT00332254). Osteoarthritis Cartilage 2012;20:271-8. [Crossref] [PubMed]
- Aman ZS, DePhillipo NN, Familiari F, et al. Acute Intervention With Selective Interleukin-1 Inhibitor Therapy May Reduce the Progression of Posttraumatic Osteoarthritis of the Knee: A Systematic Review of Current Evidence. Arthroscopy 2022;38:2543-56. [Crossref] [PubMed]
Cite this article as: Tollefson LV, Kennedy MI, Tagliero AJ, Malinowski K, Chahla J, Moatshe G, Kennedy NI, LaPrade RF, DePhillipo NN. Inflammatory synovial biomarkers and state of the tibiofemoral joint in the post-surgical settings: a narrative review. Ann Joint 2024;9:6.


