
Proximal femoral defect classifications in revision total hip arthroplasty from X-rays imaging to advanced 3D imaging: a narrative review
Introduction
The number of primary hip replacement surgeries has followed a steady increase over the years. Projections predict that in 2030 in the United States, the number of 572,000 procedures will be reached annually, leading to an increase of 175% compared to 2005 (1). With the increase of primary implant procedures, even total hip arthroplasty (THA) failures keep rising in numbers, mostly related to the progressive reduction of bone quality due to aging and consequently aseptic loosening and peri-prosthetic femoral fractures (2,3).
The management of femoral bone loss in revision hip arthroplasty represents a challenge for the orthopedic surgeon who needs to assess the residual bone stock and the altered anatomy, aiming for adequate stability of the new implants (4). Moreover, elderly patients’ comorbidities and these more demanding surgical procedures often lead to increased rates of peri and post-operative complications (5-7).
Therefore, the main goal for a “one-shot surgery” minimizing the risk of re-revision, is proper preoperative planning, which is commonly based on standard X-rays, computed tomography (CT) scans, and bone defects classification systems. Different classification systems of femoral bone defects have been proposed over the years, all aiming to quantify bone loss, describe the residual bone stock around the stem and suggest a treatment algorithm (8-14). The most widely used classification system for femoral bone defects is the Paprosky classification of 1990 (8,15).
Different studies analyzed inter-observer and intra-observer reproducibility of traditional X-ray classifications, and showing different levels of agreement varying from low to excellent (16-18). However, the description of bony defect based on traditional X-rays provides solely qualitative assessment, not providing any quantification of the bone loss, lacking in providing preoperative accurate information for preoperative planning and further analysis remains limited to intraoperative evaluation.
The utility of CT scan or 3D imaging technologies for the analysis and the classification of bone defects has been widely proven on the acetabular side (19-22). Nowadays, 3D modeling software, segmentation and metal artifacts reduction tools, in fact, allow using CT scan images and even magnetic resonance imaging (MRI) in the setting of three-dimensional (3D) bone defect assessment (23,24).
Therefore, the aim of our study is to review the current state of the art of femoral defect classification (FDC) systems in hip revision arthroplasty and highlight the role of new CT scan-based 3D modeling techniques to analyze femoral bone loss using the existing classification schemes. We present this article in accordance with the Narrative Review reporting checklist (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-47/rc).
Methods
A narrative review was conducted. The articles were selected from the PubMed and Scopus medical database updated to March 2023. The research was performed using these relevant search term and strategy items (Table 1). This was not a systematic review and does not comprehend all available literature on this topic.
Table 1
| Items | Specification |
|---|---|
| Date of search | 12 March 2023 |
| Databases and other sources searched | PubMed, Scopus |
| Search terms used | “CT scan”, “classification”, “femoral defects”, “3D”, “radiographic”, “revision hip arthroplasty” |
| Timeframe | 1970–March 2023 |
| Inclusion and exclusion criteria | Inclusion criteria: Level-I to IV studies, English language, prospective cohorts, retrospective studies, reviews, systematic reviews, accuracy or reliability (or both) studies |
| Exclusion criteria: not peer-reviewed articles; not validated classifications | |
| Selection process | Selection was conducted independently by the first author (G.M.) and other two authors (S.M.A. and A.P.) |
Our search yielded 408 results, of which 17 were deemed highly relevant. Studies in English language was considered for inclusion, with the exception for the original study regarding Endo-Klinik classification that was in German language. We found 13 studies eligible for evaluation describing X-ray-based classification systems. We did not find any of CT scan-based classification systems. Studies regarding classification systems that were not validated and were not evaluated for reproducibility by the original authors or other authors were excluded. The remaining six classifications have been described in detail in the discussion section. The emerging classifications, listed in chronologic order of publication, are listed in Table 2.
Table 2
| Classification | Author, year | Validation study |
|---|---|---|
| Endo-Klinik classification | Engelbrecht et al., 1987 | Yes |
| Mallory classification | Mallory et al., 1988 | N/A |
| Gustilo and Pasternak classification | Gustilo et al., 1988 | N/A |
| Engh classification | Engh et al., 1988 | N/A |
| Chandler and Penenberg classification | Chandler et al., 1989 | N/A |
| Johnson classification | Johnson et al., 1990 | N/A |
| Paprosky classification | Paprosky et al., 1990 | Yes |
| Aribindi et al., 1998 | ||
| Gross classification | Gross et al., 1993 | Yes |
| Saleh et al., 2001 | ||
| American Academy Orthopedic Surgeons (AAOS) classification | D’Antonio et al., 1993 | Yes |
| Parry classification | Parry et al., 2010 | Yes |
| Novel Defect System (NDS) | Rodgers et al., 2021 | N/A |
| Femoral defect classification (FDC) | Jaenisch et al., 2023 | Yes |
N/A, not available.
Discussion
Endo-Klinik classification
The Endo-Klinik’s system has been originally ideated to manage the revision of cemented stems (11). This classification system subdivides bone loss with a failed cemented femoral component into four grades. Grade 1 defects show radiolucent lines limited to the proximal half of the cement mantle in combination with clinical signs of loosening. In the second grade the medullary cavity of the proximal femur is expanded by endosteal erosion and radiolucent lines are present circumferentially. In Grade 2 defects, the proximal femur is hollowed with consequent widening of the medullary cavity and implant loosening. In Grade 4 defects, bone loss is characterized by destruction of the proximal third of the femur and the extension of the defect to the middle third not allowing even the use of a long-stemmed prosthesis (11,25). The Endo-Klinik classification is considered a simple, easy-to-apply classification system but provide low-level information and the surgeon may find patterns of bone loss that cannot be described using such a system (25). Parry et al. reported very good inter-observer (mean k score was 0.83–0.85) and intra-observer agreement (mean k score was 0.83) according to criteria of Landis and Koch (12,26). Gozzard et al. reported lower agreement for both intra-observer (mean k=0.33) and inter-observer (mean k=0.48) evaluations (16).
Paprosky classification
Paprosky et al. in 1990 proposed a classification system based on bone loss location and degree of severity, and offered for the first time a practical and worldwide approved therapeutic algorithm for surgical reconstruction (8). The original paper described four main typology of bone defects (type I, II, III and IV) and two sub-types (IIIA and IIIB). Treatment options will be discussed together with each classification category. Type I defects referred to a minimal metaphyseal bone loss, in which the proximal femoral geometry is maintained. These defects can be treated with a cylindrical porous coated stem or a tapered proximally porous-coated stem. In type II defects, femoral bone loss of the proximal metaphyseal bone has been damaged to a degree that may not be mechanically supportive for a proximally fitting stem. For this reason, a femoral implant that engages the diaphysis is typically recommended. Type III defects are described as a completely unsupportive proximal metaphysis, and the endosteal bone is severely deficient or absent. Type III defects are divided into two subcategories based on the presence of at least 4 cm of intact diaphyseal cortical bone (IIIA) or less (IIIB). In the first case, the use of a modular stem is recommended, in subtype IIIB case a modular tapered stem is preferred. In type IV defects the severe metaphyseal and diaphyseal bone loss with significant cortical thinning may lead to unreliable uncemented fixation. Treatment options include allograft prosthetic composite, cemented stem, impaction bone grafting, and cemented long stem (15).
Different authors claimed the good reliability of Paprosky classification. Brown et al. reported substantial inter-observer reliability with a k value of 0.61, and intra-observer reliability varying from k values of 0.75 to 0.81, meaning good to suboptimal agreement (17). Accordingly, Parry et al. reported its reliability with similar substantial agreement for both inter-observer (mean k=0.80) and intra-observer analysis (mean k=0.77) (12).
Nevertheless, despite the comprehensiveness and the ease in the practical application of this system, some limitations regarding the two-dimensional radiographic imaging could have been an issue to the reliability of the Paprosky classification in the assessment of bone loss (18). The group of Gozzard et al. evaluated the reproducibility of the Paprosky classification system by comparing preoperative bone loss assessment with intraoperative findings (16). Their results shown that this system as inconsistent and unreliable and may result in underestimation of bone loss.
American Academy of Orthopedic Surgeons (AAOS) classification
The AAOS classification, published in 1993 by D’Antonio et al. aimed to standardize nomenclature, assess bone defects, and support the preoperative planning (9). The system divides the femoral bone defects into six categories: segmental, cavitary, combined segmental and cavitary defect, femoral malalignment, femoral stenosis, and femoral discontinuity.
The defect/abnormality is then located into three main femoral zones: level I, proximal to the inferior portion of the lesser trochanter; level II, from the lower margin of the lesser trochanter to 10 cm distal; level III, distal to level II.
Another feature of the classification scheme is the grading of reconstructive effort needed: Grade I effort, when there’s complete prosthetic host bone contact and no bone graft is required; Grade II effort, when implant/host-bone contact is incomplete, the prosthesis is stable in host-bone and filler, or morselized graft is not needed to achieve stability but may be added to fill the void; Grade III effort, when implant/host-bone contact is incomplete, and structural bone grafting is required (such as a proximal femoral allograft).
Moreover, the authors system provided indications and a common language for surgical planning (estimation of femur bone loss with X-ray and CT scan and intraoperative inspection), highlighting the importance of templating for the choice of surgical reconstructions with both primary and revision stems. This classification revealed to be simple but did not provide any quantitative information, using only a descriptive method that does not relate directly to the magnitude of the defect or specific reconstructive options. Therefore, this classification didn’t offer a clear, reproducible therapeutic algorithm for femoral defects and its practical application has been limited. Due to its simplicity, this classification should show acceptable inter-observer and intra-observer reproducibility (18). Parry et al. reported good inter-observer agreement with a mean k score of 0.68. The mean intra-observer k score was 0.81, showing very good agreement (12). Gozzard et al., on the other hand, reported lower inter-observer (mean k=0.21) and intra-observer agreement (mean k=0.45) (16).
Gross and Saleh classification
This classification was first published by Gross et al. in 1993 (10). Then, Saleh et al. in 2001 validated the methodology and proposed a therapeutic algorithm (27). According to the authors this classification can be adapted both for cemented and uncemented implants. This system consists of five types. In type I there’s no significant loss of bone stock and the treatment options encompass conventional cemented and uncemented components. In type II there’s a contained bone loss with diaphyseal cortical thinning (the canal is widened but there is still a cortical sleeve). In these cases, the authors suggest different options for proximal fixation and distal fixation implants. Proximal fixation is achieved through impaction grafting, porous coated implant, or modular implants; when distal fixation is chosen, a long porous coated press fit implant or a long-cemented stem is proposed. In type III the defect is uncontained but proximal circumferential loss of bone stock extend for less than 5 cm in length. The treatment options are cortical strut allograft with a calcar replacing prosthesis. Type IV defects are uncontained, circumferential (more than 5 cm in length) and distal to the lesser trochanter and custom implants, tumor implants or proximal femoral allograft is needed. Type V defects comprehend periprosthetic fractures with circumferential loss of bone stock proximal to the fracture. Optimal treatment is represented by the restoration of bone stock plus long-stemmed femoral component, tumor implant or composite implant proximal femoral allograft.
According to the authors, the classification showed good inter-observer reliability (mean k=0.88) and between the blinded raters and the reference standard (intraoperative validity, k=0.87) (27). However, they later reported lower agreement between raters with a concordance between radiographic and intraoperative findings on average only 0.39 (weighted k) (28), as bone loss was rated as more severe intraoperatively while severity was underrated radiographically.
Parry classification
In 2010 the group of Parry et al. proposed a novel classification for acetabular and femoral bone loss (12). They aimed to compare the inter-observer and intra-observer reliability of the novel classification with the Paprosky classification, AAOS classification and Endo-Klinik classification. Focusing on femoral bone loss, the novel classification system describes defects as contained or uncontained and bone stock loss as minimal or significant. The defect is defined by its position as either metaphyseal or diaphyseal. Once defined the defect, the system offers a method of reconstruction that relies on the conversion of an uncontained defect to a contained defect with or without grafting to restore bone stock loss. In type A contained defect with minimal bone stock loss the reconstruction is made as a primary implant. In type B1, the defect is contained with significant bone stock loss in the metaphysis, that need to be restored by grafting. In type B2, the defect is contained with significant bone stock loss in the diaphysis, so the reconstruction is made by grafting and bypassing the defect with the implant. In type C1 the defect is uncontained with significant bone stock loss in the metaphysis and surgical plan is defined as “contain and graft”. Eventually, in type C2 the defect uncontained with significant bone stock loss in the diaphysis, therefore the reconstruction plan contemplates contain and grafting and bypass the defect. The classification scheme is simple and at the same time do not offer visual description of the defects’ subtypes. Moreover, the author didn’t provide a detailed indication about the type of implants needed for surgical reconstruction.
In their validation study, they found good intra-observer reliability (mean k=0.87) and moderate inter-observer reliability (k=0.46, k=0.73), slightly comparable to the other classification systems (12).
FDC
In 2023, a new FDC was published by the group Jaenisch et al. (13). The FDC is based on the analysis of the integrity of the main femoral segments which determine function and structural support. It focuses on the femoral neck, the metaphysis consisting of the greater and lesser trochanter, and the femoral diaphysis. This classification includes four main types of defects and numerous subcategories and provides a practical therapeutic algorithm for femoral reconstruction (Figure 1). Type 1 include defects of the femoral neck while the metaphysis remains uncompromised. In this case, cementless or cemented standard stems are recommended, or short stems if enough of the femoral neck remains.
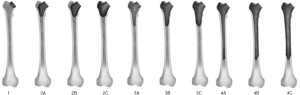
Type 2 defects involve the metaphysis. These defects are divided into three subcategories. In type 2A there’s total depletion of the metaphyseal cancellous bone, while the compact bone remains supportive on both the greater and lesser trochanter. The implant choice should be a cementless short stem, cementless or cemented standard stem. In type 2B defects, the lesser trochanter and the calcar are non-supportive in addition to the cancellous bone depletion described above. The implant choice should include a diaphyseal anchoring stem design and impaction bone grafting. In type 2C defect, the greater trochanter is disrupted in addition to the previously described defects. The implant choice should be a diaphyseal anchoring stem design in addition to impaction bone grafting and alternative bearing construct to avoid instability.
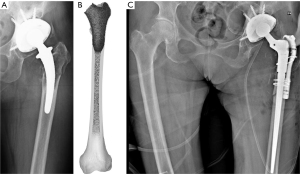
Type 3 defects involve metaphysis and diaphysis without reaching the isthmus tract. These defects are also divided into three subcategories. In type 3A defects there’s complete involvement of the cancellous bone with minimum cortical damage. The cortical offer good circumferential support. The use of a revision stem and impaction bone grafting is recommended. Type 3B defects determine complete depletion of the cancellous bone with significant damage of the cortical bone which is unsupportive <50% of the surface of this area. In these cases, the use of a revision stem in addition to impaction bone grafting and as an option diaphyseal wire/suture cerclage, is recommended. Type 3C defects comprehend complete involvement of the cancellous bone with significant damage of the cortical bone which is unsupportive >50% of the surface area. The reconstruction is based on the use of a revision stem with impaction bone grafting and a lateral strut graft in the proximal femur fixed with wire/suture cerclages (as an option: diaphyseal wire cerclages). Figure 2 shows the case of a type 3C defect in a failed short uncemented stem, and the surgical treatment according to the therapeutic algorithm.
Type 4 defects include the same patterns of type 3 defects but the isthmus femoris is compromised. In type 4A defects, there’s complete involvement of the cancellous bone with minimum cortical damage which provides good circumferential support. The implant choice should be a revision stem in addition to impaction bone grafting and strut graft and wire/suture cerclages. In type 4B the depletion of cancellous bone is associated with significant damage of the cortical bone which is unsupportive <50% of the surface. The implant choice should be a revision stem with distal screw fixation and impaction bone grafting, strut graft, and wire/suture cerclages. Type 4C defects determine the complete involvement of the cancellous bone with significant damage of the cortical bone which is unsupportive >50% of the surface. The therapeutic choice should be a proximal or total femoral replacement. The authors showed successful reproducibility of their classification method. Inter-rater reliability was good [Fleiss Kappa of 0.688; low confidence interval (CI) =0.660; high CI =0.716] and intra-rater reliability was excellent according to Landis and Koch (0.856±0.054).
CT scan-based 3D modelling for bone defect assessment
Although the previously described classifications are unanimously accepted, the lack of accuracy and reproducibility of X-rays often lead to overdiagnosis or underdiagnosis of the bone defect. Anyway, there’s no record in the literature of a CT scan-based classification for bone defect.
Different authors, highlighted the importance of using CT scans images for the preoperative planning and suggested to adapt originally X-rays based classification on CT images (19,22,29). CT scans, in fact, provide superior image clarity, more information into the 3 main plain (coronal, sagittal and axial view) and can be processed to obtain a 3-dimensional reconstruction, which are essential for preoperative planning and implant selection (22). Despite these technological advances, CT scans performed on a prosthetic joint are affected by metal artifacts that may blur the image quality definition and making almost impossible to identify the properly the tissues around the metallic devices. Their severity relies on the variety of metal types, shapes, and sizes of the implants. New commercially available metal artifact reduction (MAR) software and specific acquisition protocols allow to improve CT scan images quality, restoring the diagnostic accuracy of CT regarding the visualization of bone, bone-metal interfaces, and soft tissue structures (30).
In most complex cases, CT scan images can be elaborated using modern 3D modeling software that allow to create a digital virtual replica of the patient anatomy, splitting the metal implant from the 3D skeletal model of the hip (Figure 3) (31-33).
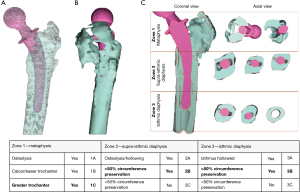
To obtain a digital replica of the patient anatomy, CT DICOM files are imported using in a 3D modeling software. First, images quality is improved using the reduction tool for metal artifacts and scattering. Then, through the process of thresholding bone tissue and stem are segmented and separate 3D objects are created. Several others step as manual contouring techniques methods can also be used to further enhance the quality of the model using a computer-aided design (CAD) software (34). Then, the model obtained can be exported in a 3D viewable design file format file (e.g., 3D pdf file) on which the analysis for the classification process is made. 3D modeling technology allows to navigate into the anatomy of the patient before the surgery, to precisely locate and quantify the bone defect and evaluate stem stability and its migration (Figure 3A,3B). The 3D object can be oriented in the coronal view corresponding to the radiographic anteroposterior (AP) view and a cross section, drawn trough to the main anatomical axis of the femur and the stem is then created. Axial sections orthogonally oriented to main axis of the femur are used to gather further information about the circumferential bone lysis at different levels corresponding to crucial supportive structures such as greater and lesser trochanter, calcar, diaphyseal cortical bone and isthmus (Figure 3C). Overall, these additional features can potentially improve the classification process even using already existing and validated X-rays classification schemes. On the 3D model, the analysis can be splitted off into three separate areas (metaphyseal, upper diaphysis and lower diaphysis) and not as unique entire-femur defect type, for a better description of the defects (Figure 3C, Table 3). Consequently, the reconstruction planning should address each different degree of bone loss found in each different zone, achieving a more precise and personalized surgery. Moreover, the model can be exported in a STL. file and then 3D printed for further analysis and a “hand-on-model” preoperative planning of the surgery.
Table 3
| Zone | Grading |
|---|---|
| Zone 1—metaphysis | |
| Osteolysis | 1A |
| Calcar/lesser trochanter | 1B |
| Greater trochanter | 1C |
| Zone 2—supra-isthmic diaphysis | |
| Osteolysis/hollowing | 2A |
| >50% circumference preservation | 2B |
| <50% circumference preservation | 2C |
| Zone 3—isthmic diaphysis | |
| Isthmus hollowed | 3A |
| >50% circumference preservation | 3B |
| <50% circumference preservation | 3C |
Adaptation of FDC. The three main zones are individually analyzed and determine the location of the defect while subcategories A, B and C are being used to classify the extent of damage in each location. The case shown in Figure 3 is reported as a subtype C in Zone 1, a subtype B in Zone 2 and Zone 3. 3D, three-dimensional; FDC, femoral defect classification.
Conclusions
This review summarizes the current literature on the proximal FDCs, focusing on the rationale of the most used systems. Accuracy and reproducibility were analyzed along with the clinical applications and treatment algorithms. Several X-rays-based classifications methods for femoral bone defects have been proposed since the 1980’s. Endo-Klinik, AAOS and Paprosky classification systems have been used the most in the last decades. The Endo-Klinik’s system has been structured to evaluate mostly the option of cemented revision arthroplasty while the other classification systems were based on diaphyseal implantation of a cementless stem. The system has been demonstrated to be reproducible but it fails to identify differences in bone loss patterns. Paprosky classification is the most used system in current clinical practice because of its detailed pattern analysis, its reproducibility, and the worldwide approved therapeutic algorithm. The AAOS introduced an extensive classification describing the full spectrum of femoral defects found in primary and revision hip arthroplasty, however, this system fails to quantify the magnitude of the defect and does not provide a clear therapeutic algorithm. FDC is new X-ray-based FDC system that unify the most relevant key concepts of the previous classifications. They highlighted the crucial role of the supportive bone structures and subdivided the femur into four main zones according to the different primary stem designs to revise. The system also revealed to be reliable, with excellent agreement between preoperative radiographs and intraoperative findings.
However, classification systems are still based on two-dimensional imaging. X-rays showed inadequacy in quantify bone loss and the residual bone stock, mostly determining underrating or overrating of the lesion.
Over the years, new diagnostic technologies spread in the orthopedic field. 3D modeling and 3D printing based on CT scan imaging represent a modern tool for better understand and manage bone loss, as several studies have reported the application of acetabular defects classification on a digital real-size anatomical replica. Bone defects can be better described, located, and quantified using 3D modeling techniques, even improving, and adapting classification schemes originally created for two-dimensional imaging techniques. The main advantages include more accurate analysis of bone loss and pre-operative planning, potentially traducing in time sparing and less complications. Therefore, future research efforts should address the need for a new 3D-based FDC and a “precision surgery based” treatment algorithm. There are some limitations to this narrative review. The search was performed only on two databases and was limited to the most relevant articles using select keywords. Articles included were mostly retrospective studies and the quality of the studies referenced was not assessed using a standardized methodology. In the future a meta-analyses and systematic review should address this topic including new classification systems.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Modular Implants for Revision Arthroplasty in Orthopedics”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-47/rc
Peer Review File: Available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-47/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoj.amegroups.com/article/view/10.21037/aoj-23-47/coif). The series “Modular Implants for Revision Arthroplasty in Orthopedics” was commissioned by the editorial office without any funding or sponsorship. G.M. served as the unpaid Guest Editor of the series. D.K. serves as an unpaid editorial board member of Annals of Joint from April 2022 to March 2024. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [Crossref] [PubMed]
- Abdel MP, Watts CD, Houdek MT, et al. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J 2016;98-B:461-7. [Crossref] [PubMed]
- Torre marina, carrani eugenio, ceccarelli stefania, biondi alessia, masciocchi mascia, cornacchia attanasio. Registro Italiano ArtroProtesi. Report Annuale 2019. Roma: Il Pensiero Scientifico Editore; 2020.
- Sadoghi P, Liebensteiner M, Agreiter M, et al. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty 2013;28:1329-32. [Crossref] [PubMed]
- Streubel PN. Mortality after periprosthetic femur fractures. J Knee Surg 2013;26:27-30. [Crossref] [PubMed]
- Capone A, Congia S, Civinini R, et al. Periprosthetic fractures: epidemiology and current treatment. Clin Cases Miner Bone Metab 2017;14:189-96. [Crossref] [PubMed]
- Rollo G, Logroscino G, Stomeo D, et al. Comparing the use of preformed vs hand-made antibiotic spacer cement in two stages revision of hip periprosthetic infection. J Clin Orthop Trauma 2020;11:S772-8. [Crossref] [PubMed]
- Paprosky W, Lawrence J, Cameron H. Femoral defect classification: clinical application. Orthop Rev 1990;19:9-17.
- D'Antonio J, McCarthy JC, Bargar WL, et al. Classification of femoral abnormalities in total hip arthroplasty. Clin Orthop Relat Res 1993;133-9. [Crossref] [PubMed]
- Gross AE, Allan DG, Lavoie GJ, et al. Revision arthroplasty of the proximal femur using allograft bone. Orthop Clin North Am 1993;24:705-15. [Crossref] [PubMed]
- Engelbrecht E, Heirnert K. Klassification und Behandlungsrichtlinien von Knochensubstanzverlustenbei Revisionsoperationen am Huftgelenk-mittelfristige Ergebnisse. Primare und Revisionsalloarthroplastik. Berlin Springer-Verlag; 1987:189-20.
- Parry MC, Whitehouse MR, Mehendale SA, et al. A comparison of the validity and reliability of established bone stock loss classification systems and the proposal of a novel classification system. Hip Int 2010;20:50-5. [Crossref] [PubMed]
- Jaenisch M, Kohlhof H, Kasapovic A, et al. Femoral defects in revision hip arthroplasty: a therapy-oriented classification. Arch Orthop Trauma Surg 2023;143:1163-74. [Crossref] [PubMed]
- Rodgers B, Wernick G, Roman G, et al. A Contemporary Classification System of Femoral Bone Loss in Revision Total Hip Arthroplasty. Arthroplast Today 2021;9:134-40. [Crossref] [PubMed]
- Aribindi R, Barba M, Solomon MI, et al. Bypass fixation. Orthop Clin North Am 1998;29:319-29. [Crossref] [PubMed]
- Gozzard C, Blom A, Taylor A, et al. A comparison of the reliability and validity of bone stock loss classification systems used for revision hip surgery. J Arthroplasty 2003;18:638-42. [Crossref] [PubMed]
- Brown NM, Foran JR, Valle CJ, et al. The inter-observer and intra-observer reliability of the Paprosky femoral bone loss classification system. J Arthroplasty 2014;29:1482-4. [Crossref] [PubMed]
- Gu A, Adriani M, Malahias MA, et al. Reliability and Validity of Acetabular and Femoral Bone Loss Classification Systems in Total Hip Arthroplasty: A Systematic Review. HSS J 2020;16:288-95. [Crossref] [PubMed]
- Meynen A, Vles G, Roussot M, et al. Advanced quantitative 3D imaging improves the reliability of the classification of acetabular defects. Arch Orthop Trauma Surg 2023;143:1611-7. [Crossref] [PubMed]
- Gelaude F, Clijmans T, Delport H. Quantitative Computerized Assessment of the Degree of Acetabular Bone Deficiency: Total radial Acetabular Bone Loss (TrABL). Adv Orthop 2011;2011:494382. [Crossref] [PubMed]
- Marongiu G, Prost R, Capone A. A New Diagnostic Approach for Periprosthetic Acetabular Fractures Based on 3D Modeling: A Study Protocol. Diagnostics (Basel) 2019;10:15. [Crossref] [PubMed]
- Horas K, Arnholdt J, Steinert AF, et al. Acetabular defect classification in times of 3D imaging and patient-specific treatment protocols. Orthopade 2017;46:168-78. [Crossref] [PubMed]
- Gupta A, Subhas N, Primak AN, et al. Metal artifact reduction: standard and advanced magnetic resonance and computed tomography techniques. Radiol Clin North Am 2015;53:531-47. [Crossref] [PubMed]
- Wellenberg RHH, Hakvoort ET, Slump CH, et al. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur J Radiol 2018;107:60-9. [Crossref] [PubMed]
- Masri BA, Masterson EL, Duncan CP. The classification and radiographic evaluation of bone loss in revision hip arthroplasty. Orthop Clin North Am 1998;29:219-27. [Crossref] [PubMed]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Saleh KJ, Holtzman J. Development, test reliability and validation of a classification for revision hip arthroplasty. J Orthop Res 2001;19:50-6. [Crossref] [PubMed]
- Davis AM, Schemitsch EH, Gollish JD, et al. Classifying failed hip arthroplasty: generalizability of reliability and validity. Clin Orthop Relat Res 2003;171-9. [Crossref] [PubMed]
- Sakellariou VI, Babis GC. Management bone loss of the proximal femur in revision hip arthroplasty: Update on reconstructive options. World J Orthop 2014;5:614-22. [Crossref] [PubMed]
- Marongiu G, Prost R, Capone A. Use of 3D modelling and 3D printing for the diagnostic process, decision making and preoperative planning of periprosthetic acetabular fractures. BMJ Case Rep 2020;13:e233117. [Crossref] [PubMed]
- Salazar DA, Cramer J, Markin NW, et al. Comparison of 3D printed anatomical model qualities in acetabular fracture representation. Ann Transl Med 2022;10:391. [Crossref] [PubMed]
- Aprato A, Olivero M, Iannizzi G, et al. Pelvic discontinuity in acetabular revisions: does CT scan overestimate it? A comparative study of diagnostic accuracy of 3D-modeling and traditional 3D CT scan. Musculoskelet Surg 2020;104:171-7. [Crossref] [PubMed]
- Ballard DH, Mills P, Duszak R Jr, et al. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad Radiol 2020;27:1103-13. [Crossref] [PubMed]
- Mandolini M, Brunzini A, Facco G, et al. Comparison of Three 3D Segmentation Software Tools for Hip Surgical Planning. Sensors (Basel) 2022;22:5242. [Crossref] [PubMed]
Cite this article as: Marongiu G, Leinardi L, Antuofermo SM, Pili A, Verona M, Kendoff D, Zampogna B, Capone A. Proximal femoral defect classifications in revision total hip arthroplasty from X-rays imaging to advanced 3D imaging: a narrative review. Ann Joint 2024;9:18.


